| Revision as of 09:21, 6 December 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 464303919 of page Potassium_hydroxide for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 09:22, 6 December 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 464303031 of page Sodium_carbonate for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 458281776 | ||
| | Name = Potassium hydroxide | |||
| | Name = Sodium carbonate | |||
| | ImageFile = |
| ImageFile = Sodium carbonate.png | ||
| | ImageSize = |
| ImageSize = 180px | ||
| | ImageName = pellets of potassium hydroxide | |||
| | ImageName = Structural formula of sodium carbonate | |||
| | ImageFile1 = Potassium-hydroxide-xtal-3D-vdW.png | |||
| | ImageFile1 = Uhličitan sodný.JPG | |||
| | ImageSize1 = 200px | |||
| | ImageName1 = Sodium carbonate | |||
| | ImageName1 = crystal structure of KOH | |||
| | ImageFile2 = Sodium-carbonate-xtal-3D-SF-C.png | |||
| | IUPACName = Potassium hydroxide | |||
| | ImageName2 = Space-filling model of the crystal structure of sodium carbonate | |||
| | OtherNames = Caustic potash<br/>Potash lye<br/>Potassia<br/>Potassium hydrate | |||
| | OtherNames = Soda ash<br/>Washing soda<br/>Soda crystals | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| ⚫ | | CASNo = 497-19-8 | ||
| ⚫ | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | UNII = |
||
| | CASOther = <br>5968-11-6 (monohydrate)<br>6132-02-1 (decahydrate) | |||
| | InChI = 1/K.H2O/h;1H2/q+1;/p-1 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | InChIKey = KWYUFKZDYYNOTN-REWHXWOFAT | |||
| | ChEMBL = 186314 | |||
| ⚫ | | PubChem = 10340 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 9916 | ||
| ⚫ | | RTECS = VZ4050000 | ||
| ⚫ | | EINECS = 207-838-8| UNII_Ref = {{fdacite|correct|FDA}} | ||
| ⚫ | | UNII = 45P3261C7T | ||
| | InChI = 1/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | |||
| | InChIKey = CDBYLPFSWZWCQE-NUQVWONBAP | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = |
| ChEBI = 29377 | ||
| | SMILES = . | | SMILES = ..C()=O | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = CDBYLPFSWZWCQE-UHFFFAOYSA-L}} | ||
| ⚫ | | CASNo = |
||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | PubChem = |
||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = |
||
| ⚫ | | RTECS = |
||
| | UNNumber = 1813 | |||
| | EINECS = 215-181-3 | |||
| ⚫ | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = |
| Formula = Na<sub>2</sub>CO<sub>3</sub> | ||
| | MolarMass = 105.9784 g/mol (anhydrous) <br> 124.00 g/mol (monohydrate) <br> 286.14 g/mol (decahydrate) | |||
| | MolarMass = 56.1056 g/mol | |||
| | Appearance = |
| Appearance = White solid, ] | ||
| | Odor = Odorless | |||
| | Density = 2.044 g/cm<sup>3</sup><ref name=crc/> | |||
| | Density = 2.54 g/cm<sup>3</sup> (anhydrous) <br> 2.25 g/cm<sup>3</sup> (monohydrate) <br> 1.46 g/cm<sup>3</sup> (decahydrate) | |||
| | Solubility = 1210 g/L (25 °C) <br> 1780 g/L (100 °C)<ref name=crc>{{RubberBible86th|page=4-80}}</ref> | |||
| | Solubility = 70 g/L (0 °C)<br/>216 g/L (20 °C)<ref name=UNEP>{{cite web|publisher = UNEP Publications|url = http://www.chem.unep.ch/irptc/sids/oecdsids/Naco.pdf|title = Sodium Carbonate}}</ref><br/>450 g/L (100 °C)<ref name="ndctz.com">{{dead link|date=June 2011}}</ref> | |||
| | SolubleOther = soluble in ], ] <br> insoluble in ], liquid ] | |||
| | |
| HeatofSolution = 24.7 kJ/mol at 100 mol H<sub>2</sub>O<ref name="ndctz.com"/> | ||
| | SolubleOther = insoluble in ] | |||
| ⚫ | | BoilingPt = |
||
| | MeltingPt = 851 °C (anhydrous)<ref name=UNEP>{{cite web|publisher = UNEP Publications|url = http://www.chem.unep.ch/irptc/sids/oecdsids/Naco.pdf|title = Sodium Carbonate}}</ref> <br> 100 °C (decomp, monohydrate) <br> 34 °C (decomp, decahydrate) | |||
| | pKa = 13.5 (0.1 M) | |||
| ⚫ | | BoilingPt = 1633 °C (anhydrous) | ||
| | RefractIndex = 1.409 | |||
| | pKb = 4.67 | |||
| ⚫ | }} | ||
| | RefractIndex = 1.485 (anhydrous) <br> 1.420 (monohydrate) | |||
| ⚫ | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | Coordination = | | Coordination = trigonal planar | ||
| | Structure = triclinic (anhydrous) <br> orthorhombic (monohydrate) | |||
| | CrystalStruct =rhombohedral | |||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | ExternalMSDS = | | ExternalMSDS = | ||
| | EUIndex = |
| EUIndex = 011-005-00-2 | ||
| | EUClass = |
| EUClass = Irritant ('''Xi''') | ||
| ⚫ | | NFPA-H = 1 | ||
| | RPhrases = {{R22}}, {{R35}} | |||
| ⚫ | | NFPA-F = 0 | ||
| ⚫ | | SPhrases = {{ |
||
| | NFPA- |
| NFPA-R = 1 | ||
| | |
| RPhrases = {{R36}} | ||
| ⚫ | | SPhrases = {{S2}}, {{S22}}, {{S26}} | ||
| ⚫ | | NFPA- |
||
| ⚫ | | FlashPt = Non-flammable | ||
| ⚫ | | NFPA- |
||
| ⚫ | }} | ||
| ⚫ | | FlashPt = Non-flammable | ||
| | LD50 = 273 mg/kg | |||
| }} | |||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | OtherAnions = ]<br/>] | | OtherAnions = ] | ||
| | OtherCations = ]<br/>]<br/>]<br/>] | | OtherCations = ]<br/>]<br/>]<br/>] | ||
| | OtherCpds = ] | | OtherCpds = ]<br/>]<br/>] | ||
| }} | }} | ||
| }} | }} | ||
Revision as of 09:22, 6 December 2011
| This page contains a copy of the infobox ({{chembox}}) taken from revid 464303031 of page Sodium_carbonate with values updated to verified values. |
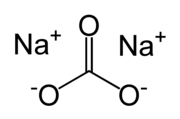
| |

| |
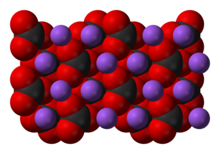
| |
| Names | |
|---|---|
| Other names
Soda ash Washing soda Soda crystals | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Na2CO3 |
| Molar mass | 105.9784 g/mol (anhydrous) 124.00 g/mol (monohydrate) 286.14 g/mol (decahydrate) |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density | 2.54 g/cm (anhydrous) 2.25 g/cm (monohydrate) 1.46 g/cm (decahydrate) |
| Melting point | 851 °C (anhydrous) 100 °C (decomp, monohydrate) 34 °C (decomp, decahydrate) |
| Boiling point | 1633 °C (anhydrous) |
| Solubility in water | 70 g/L (0 °C) 216 g/L (20 °C) 450 g/L (100 °C) |
| Solubility | insoluble in ethanol |
| Basicity (pKb) | 4.67 |
| Refractive index (nD) | 1.485 (anhydrous) 1.420 (monohydrate) |
| Structure | |
| Coordination geometry | trigonal planar |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Sodium bicarbonate |
| Other cations | Lithium carbonate Potassium carbonate Rubidium carbonate Caesium carbonate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chemical compound
- ^ "Sodium Carbonate" (PDF). UNEP Publications.
- ^