| Revision as of 09:26, 6 December 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 464298351 of page Sodium_fluoride for the Chem/Drugbox validation project (updated: 'KEGG').← Previous edit | Revision as of 09:26, 6 December 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 464263360 of page Sodium_bicarbonate for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{Chembox | {{Chembox | ||
| | verifiedrevid = 464184701 | |||
| | Verifiedfields = changed | |||
| | ImageFile2 = Sodium bicarbonate.jpg | |||
| | verifiedrevid = 458956980 | |||
| | ImageFile2_Ref = {{Chemboximage|correct|??}} | |||
| | Name = Sodium fluoride | |||
| | ImageSize2 = 244 | |||
| | ImageFile = Sodium-fluoride-3D-ionic.png | |||
| | ImageName2 = Sample of sodium bicarbonate | |||
| <!-- | ImageSize = 150px --> | |||
| | ImageFile = SodiumBicarbonate.svg | |||
| | ImageName = Sodium fluoride | |||
| | ImageFile_Ref = {{Chemboximage|correct|??}} | |||
| | IUPACName = Sodium fluoride | |||
| | ImageSize = 121 | |||
| | OtherNames = Florocid | |||
| | ImageFileL1 = Sodium-3D.png | |||
| | ImageFileL1_Ref = {{Chemboximage|correct|??}} | |||
| | ImageSizeL1 = 121 | |||
| | ImageNameL1 = Ball and stick model of a sodium cation | |||
| | ImageFileR1 = Bicarbonate-ion-3D-balls-B.png | |||
| | ImageFileR1_Ref = {{Chemboximage|correct|??}} | |||
| | ImageSizeR1 = 121 | |||
| | ImageNameR1 = Ball and stick model of a bicarbonate anion | |||
| | IUPACName = Sodium hydrogen carbonate | |||
| | SytematicName = <!-- Sodium hydrogen carbonate --> | |||
| | OtherNames = Baking soda, bicarbonate of soda, ], sodium bicarbonate, sodium hydrogencarbonate | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | CASNo = 144-55-8 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | ChemSpiderID = 5045 | |||
| | PubChem = 516892 | |||
| | PubChem_Ref = {{Pubchemcite|correct|??}} | |||
| | ChemSpiderID = 8609 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | UNII = 8MDF5V39QO | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | |
| EINECS = 205-633-8 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | InChI = 1/FH.Na/h1H;/q;+1/p-1 | |||
| | DrugBank = DB01390 | |||
| | InChIKey = PUZPDOWCWNUUKD-REWHXWOFAH | |||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C12603 | |||
| | StdInChIKey = PUZPDOWCWNUUKD-UHFFFAOYSA-M | |||
| | MeSHName = Sodium+bicarbonate | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 32139 | |||
| | ChEMBL = 1353 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | |
| RTECS = VZ0950000 | ||
| | ATCCode_prefix = B05 | |||
| | CASNo = 7681-49-4 | |||
| | ATCCode_suffix = CB04 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | ATC_Supplemental = {{ATC|B05|XA02}} | |||
| | RTECS = WB0350000 | |||
| | |
| Beilstein = 4153970 | ||
| | SMILES = .OC()=O | |||
| | UNNumber = 1690 | |||
| | StdInChI = 1S/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 28741 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
| InChI = 1/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 | ||
| | StdInChIKey = UIIMBOGNXHQVGW-UHFFFAOYSA-M | |||
| | PubChem = 5235 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | ATCCode_prefix=A01 | |||
| | InChIKey = UIIMBOGNXHQVGW-REWHXWOFAQ}} | |||
| | ATCCode_suffix=AA01 | |||
| | KEGG_Ref = {{keggcite|changed|kegg}} | |||
| | KEGG = <!-- blanked - oldvalue: C08142 --> | |||
| }} | |||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| C = 1 | ||
| | H = 1 | |||
| | MolarMass = 41.988713 g/mol | |||
| | Na = 1 | |||
| | Appearance = White solid | |||
| | |
| O = 3 | ||
| | |
| ExactMass = 83.982338573 g mol<sup>−1</sup> | ||
| | Appearance = White crystals | |||
| | Solubility = 0.5 M (20 °C) | |||
| | Density = 2.20 g cm<sup>−3</sup><ref name="crc84.4-85">"Physical Constants of Inorganic Compounds". ''CRC Handbook'', p. 4-85.</ref> | |||
| | SolubleOther = soluble in ] <br> insoluble in ] | |||
| | |
| MeltingPtC = 50 | ||
| | |
| BoilingPtC = 851 | ||
| | Melting_notes = decomposes | |||
| | VaporPressure = 1 mmHg @ 1077 C°<ref>Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 10th ed. Volumes 1-3 New York, NY: John Wiley & Sons Inc., 1999., p. 3248</ref> | |||
| | Solubility = 9 g/100 mL | |||
| }} | |||
| 69 g/L (0 °C)<ref name=crc84.8-116>"Aqueous solubility of inorganic compounds at various temperatures". ''CRC Handbook'', p. 8-116.</ref> <br> | |||
| | Section7 = {{Chembox Hazards | |||
| 96 g/l (20 °C)<ref name=UNEP>{{cite web|publisher = UNEP Publications|url = http://www.chem.unep.ch/irptc/sids/oecdsids/Sodium%20bicarbonate.pdf|title = Sodium Bicarbonate}}</ref> <br> | |||
| | ExternalMSDS = | |||
| 165 g/l (60 °C)<ref name=UNEP/><br> | |||
| | EUIndex = 009-004-00-7 | |||
| 236 g/L (100 °C)<ref name=crc84.8-116/> | |||
| | EUClass = Toxic ('''T''')<br/>Irritant ('''Xi''') | |||
| | LogP = -0.82 | |||
| | RPhrases = {{R25}}, {{R32}}, {{R36/38}} | |||
| | pKa = 10.329<ref name="crc84.7-13">{{cite book|last1=Goldberg|first1=Robert N.|last2=Kishore|first2=Nand|last3=Lennen|first3=Rebecca M.|title=CRC Handbook|pages=7–13|contribution=Thermodynamic quantities for the ionization reactions of buffers in water}}</ref> | |||
| | SPhrases = {{S1/2}}, {{S22}}, {{S36}}, {{S45}} | |||
| 6.351 (carbonic acid)<ref name=crc84.7-13/> | |||
| | NFPA-H = 3 | |||
| | RefractIndex = 1.3344}} | |||
| | NFPA-F = 0 | |||
| | Section3 = {{Chembox Pharmacology | |||
| | NFPA-R = 0 | |||
| | AdminRoutes = Intravenous, oral}} | |||
| | FlashPt = Non-flammable | |||
| | Section4 = {{Chembox Hazards | |||
| | LD50 = 52–200 mg/kg (oral in rats, mice, rabbits)<ref>{{Citation |author=Martel, B.; Cassidy, K. |title=Chemical Risk Analysis: A Practical Handbook |publisher=Butterworth–Heinemann |year=2004 |isbn=1903996651 |page=363}}</ref> | |||
| | ExternalMSDS = | |||
| }} | |||
| | MainHazards = Causes serious eye irritation | |||
| | NFPA-H = 1 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | LD50 = 4.22 g kg<sup>−</sup>}} | |||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherAnions = ] | ||
| | |
| OtherCations = ]<br /> | ||
| ] | |||
| | OtherCpds = ] | |||
| | OtherCpds = ]<br /> | |||
| }} | |||
| ]}} | |||
| }} | }} | ||
Revision as of 09:26, 6 December 2011
| This page contains a copy of the infobox ({{chembox}}) taken from revid 464263360 of page Sodium_bicarbonate with values updated to verified values. |
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium hydrogen carbonate | |||
| Other names Baking soda, bicarbonate of soda, nahcolite, sodium bicarbonate, sodium hydrogencarbonate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 4153970 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| KEGG | |||
| MeSH | Sodium+bicarbonate | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
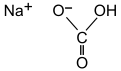
| Chemical formula | CHNaO3 | ||
| Molar mass | 84.006 g·mol | ||
| Appearance | White crystals | ||
| Density | 2.20 g cm | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| Boiling point | 851 °C (1,564 °F; 1,124 K) | ||
| Solubility in water | 9 g/100 mL
69 g/L (0 °C) | ||
| log P | -0.82 | ||
| Acidity (pKa) | 10.329
6.351 (carbonic acid) | ||
| Refractive index (nD) | 1.3344 | ||
| Pharmacology | |||
| Routes of administration |
Intravenous, oral | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Causes serious eye irritation | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 4.22 g kg | ||
| Related compounds | |||
| Other anions | Sodium carbonate | ||
| Other cations | Ammonium bicarbonate | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- "Physical Constants of Inorganic Compounds". CRC Handbook, p. 4-85.
- ^ "Aqueous solubility of inorganic compounds at various temperatures". CRC Handbook, p. 8-116.
- ^ "Sodium Bicarbonate" (PDF). UNEP Publications.
- ^ Goldberg, Robert N.; Kishore, Nand; Lennen, Rebecca M. "Thermodynamic quantities for the ionization reactions of buffers in water". CRC Handbook. pp. 7–13.

