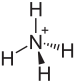| Revision as of 12:17, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476519550 of page Cocamidopropyl_betaine for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 12:17, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476504368 of page Ammonium_bisulfate for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{chembox | ||
| ⚫ | | verifiedrevid = 419603257 | ||
| | Verifiedfields = changed | |||
| | ImageFileL1 = Ammonium.svg | |||
| | Watchedfields = changed | |||
| | ImageSizeL1 = 75 | |||
| ⚫ | | verifiedrevid = |
||
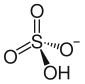
| | ImageFileR1 = Hydrogen sulfate.svg | |||
| | Name = Lauramidopropyl betaine | |||
| | ImageSizeR1 = 85 | |||
| | ImageFile = Cocamidopropyl betaine2.png | |||
| | ImageFile2 = Ammonium-bisulfate-3D-balls.png | |||
| ⚫ | | |
||
| | ImageName2 = Ball-and-stick model of an ammonium cation (left) and a bisulfite anion (right) | |||
| | ImageSize = 244 | |||
| | IUPACName = Ammonium hydrogen sulfate | |||
| | ImageName = Structural formula of lauramidopropyl betaine | |||
| | OtherNames = | |||
| | ImageCaption = Lauramidopropyl betaine, the major component of cocamidopropyl betaine | |||
| | IUPACName = {(dimethyl)ammonio}acetate | |||
| | SystematicName = <!-- 2-acetate (substitutive) OR (Carboxylatomethyl)(3-dodecanamidopropyl)dimethylnitrogen (additive) --> | |||
| | OtherNames = 2-acetate | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| ⚫ | | |
||
| | CASNo_Ref = {{cascite|changed|??}} | |||
| ⚫ | | PubChem = |
||
| | PubChem_Ref = {{Pubchemcite|correct|PubChem}} | |||
| ⚫ | | |
||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 23057 | ||
| | EINECS = 263-058-8 | |||
| | InChI = 1/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4) | |||
| | SMILES = CCCCCCCCCCCC(=O)NCCC(C)(C)CC()=O | |||
| | |
| SMILES = S(=O)(=O)O. | ||
| | InChIKey = BIGPRXCJEDHCLP-UHFFFAOYAA | |||
| | SMILES2 = C(=O)C(CCCNC(=O)CCCCCCCCCCC)(C)C | |||
| ⚫ | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C19H38N2O3/c1-4-5-6-7-8-9-10-11-12-14-18(22)20-15-13-16-21(2,3)17-19(23)24/h4-17H2,1-3H3,(H-,20,22,23,24) | |||
| | StdInChI = 1S/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4) | |||
| ⚫ | | |
||
| ⚫ | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | InChI = 1/C19H38N2O3/c1-4-5-6-7-8-9-10-11-12-14-18(22)20-15-13-16-21(2,3)17-19(23)24/h4-17H2,1-3H3,(H-,20,22,23,24) | |||
| | |
| StdInChIKey = BIGPRXCJEDHCLP-UHFFFAOYSA-N | ||
| ⚫ | | CASNo = 7803-63-6 | ||
| ⚫ | | |
||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | InChIKey = MRUAUOIMASANKQ-UHFFFAOYAL}} | |||
| ⚫ | | PubChem = 16211166 | ||
| | RTECS = WS990000 | |||
| }} | |||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = (NH<sub>4</sub>)HSO<sub>4</sub> | |||
| | C = 19 | |||
| | |
| MolarMass = 115.11 g/mol | ||
| | |
| Appearance = White solid | ||
| | Density = 1.78 g/cm<sup>3</sup> | |||
| | O = 3 | |||
| | MeltingPtC = 147 | |||
| | ExactMass = 342.288243092 g/mol}} | |||
| | BoilingPt = | |||
| | Solubility = Very soluble | |||
| | SolubleOther = Soluble in ] <br> insoluble in ] | |||
| | Solvent = other solvents | |||
| }} | |||
| | Section3 = {{Chembox Hazards | |||
| | EUIndex = Not listed | |||
| | ExternalMSDS = | |||
| | FlashPt = | |||
| | Autoignition = | |||
| | NFPA-H = 3 | NFPA-F = 0 | NFPA-R = 0 | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | OtherAnions = ]<br/>]<br/>]<br/>] | |||
| | OtherCations = ]<br/>] | |||
| | OtherCpds = | |||
| }} | |||
| }} | }} | ||
Revision as of 12:17, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 476504368 of page Ammonium_bisulfate with values updated to verified values. |
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Ammonium hydrogen sulfate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| RTECS number |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | (NH4)HSO4 | ||
| Molar mass | 115.11 g/mol | ||
| Appearance | White solid | ||
| Density | 1.78 g/cm | ||
| Melting point | 147 °C (297 °F; 420 K) | ||
| Solubility in water | Very soluble | ||
| Solubility in other solvents | Soluble in methanol insoluble in acetone | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Other anions | Ammonium thiosulfate Ammonium sulfite Ammonium sulfate Ammonium persulfate | ||
| Other cations | Sodium bisulfate Potassium bisulfate | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound