| Revision as of 12:28, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,044 edits Saving copy of the {{chembox}} taken from revid 476446124 of page Potassium_chloride for the Chem/Drugbox validation project (updated: 'ChEMBL').← Previous edit | Revision as of 12:29, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,044 edits Saving copy of the {{chembox}} taken from revid 475232701 of page Tartaric_acid for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | |
| Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 470481910 | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| | ImageFile = |
| ImageFile = Tartaric acid.svg | ||
| | ImageFile1 = |
| ImageFile1 = Tartaric-acid-3D-balls.png | ||
| | OtherNames = ]<br/>Muriate of potash | |||
| | ImageSize = | |||
| | IUPACName = 2,3-dihydroxybutanedioic acid | |||
| | OtherNames = 2,3-dihydroxysuccinic acid<br>threaric acid<br>racemic acid<br>uvic acid<br>paratartaric acid | |||
| | Reference = <ref>, ].</ref> | |||
| |- | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | |
| KEGG = C00898 | ||
| | InChI = 1/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10) | |||
| ⚫ | | |
||
| | InChIKey = FEWJPZIEWOKRBE-UHFFFAOYAZ | |||
| | ChEMBL = <!-- blanked - oldvalue: 1200731 --> | |||
| | |
| ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | |
| ChEMBL = 333714 | ||
| | ChEMBL2 = 1200861 | |||
| | InChI = 1/ClH.K/h1H;/q;+1/p-1 | |||
| | InChIKey = WCUXLLCKKVVCTQ-REWHXWOFAZ | |||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| ⚫ | | DrugBank = |
||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ⚫ | | ChEBI = |
||
| | SMILES = . | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10) | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = FEWJPZIEWOKRBE-UHFFFAOYSA-N | ||
| ⚫ | | CASNo = |
||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | CASNo = 526-83-0 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
| PubChem = 875 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | PubChem = 4873 | |||
| | ChemSpiderID = 852 | |||
| | RTECS = TS8050000 | |||
| | MeSHName = tartaric+acid | |||
| | ATCCode_prefix = A12 | |||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | ATCCode_suffix = BA01 | |||
| ⚫ | | DrugBank = DB01694 | ||
| | ATC_Supplemental = {{ATC|B05|XA01}} | |||
| ⚫ | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| ⚫ | | ChEBI = 15674 | ||
| | SMILES = O=C(O)C(O)C(O)C(=O)O | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = C<sub>4</sub>H<sub>6</sub>O<sub>6</sub><small> (Basic formula)</small><br>HO<sub>2</sub>CCH(OH)CH(OH)CO<sub>2</sub>H <small>(Structural formula)</small> | |||
| | Formula = KCl | |||
| | MolarMass = |
| MolarMass = 150.087 g/mol | ||
| | Appearance = white |
| Appearance = white powder | ||
| ⚫ | | Density = 1.79 g/mL (H<sub>2</sub>O) | ||
| | Odor = odorless | |||
| | MeltingPt = 171–174 °C (''L or D''-tartaric; pure)<br>206 °C (''DL'', racemic)<br> 165-166°C ("meso-anhyrdous") <br> 146–148 °C (''meso-hydrous'')<ref>{{RubberBible86th}}</ref> <br> | |||
| ⚫ | | Density = 1. |
||
| ⚫ | | BoilingPt = | ||
| | Solubility = 281 g/L (0°C) <br> 344 g/L (20°C) <br> 567 g/L (100°C) | |||
| | Solubility = 133 g/100ml (20 °C) | |||
| | SolubleOther = soluble in ], ]es <br> slightly soluble in ], insoluble in ]<ref>{{cite web | url = http://www.inchem.org/documents/pims/pharm/potasscl.htm | title = Potassium chloride (PIM 430) | at = 3.3.1 Properties of the substance | publisher = ] | accessdate = 2011-01-17 }}</ref> | |||
| | pKa = L(+) 25 °C :<br>pK<sub>a1</sub>= 2.95 pK<sub>a2</sub>= 4.25<br>meso 25 °C:<br>pK<sub>a1</sub>= 3.22 pK<sub>a2</sub>= 4.85 | |||
| | MeltingPt = 770 °C | |||
| ⚫ | | BoilingPt = |
||
| | RefractIndex = 1.4902 (589 nm) | |||
| | pKa = ~7 | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | CrystalStruct = ] | |||
| }} | }} | ||
| | |
| Section3 = {{Chembox Hazards | ||
| | MainHazards = | |||
| | DeltaHf = −436 kJ·mol<sup>−1</sup><ref name=b1>{{cite book| author = Zumdahl, Steven S.|title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 061894690X|page=A22}}</ref> | |||
| |EUClass=Irritant('''Xi''') | |||
| | Entropy = 83 J·mol<sup>−1</sup>·K<sup>−1</sup><ref name=b1/> | |||
| |RPhrases={{R36}} | |||
| }} | |||
| | Autoignition = | |||
| | Section7 = {{Chembox Hazards | |||
| | ExternalMSDS = | |||
| | EUIndex = Not listed | |||
| | FlashPt = Non-flammable | |||
| | NFPA-H = 1 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | NFPA-O = | |||
| | LD50 = 2.6 g/kg (oral/rat), 0.142 g/kg (intravenous/rat)<ref>{{Cite book|title = Material Safety Data Sheet – Potassium Chloride|publisher = Sigma–Aldrich|date = July 2001}}</ref> | |||
| }} | }} | ||
| | |
| Section4 = {{Chembox Related | ||
| | |
| OtherCations = ]<br />]<br />]<br />] | ||
| | Function = ]s | |||
| | OtherCations = ]<br/>]<br/>]<br/>] | |||
| | OtherFunctn = ]<br />]<br />]<br />]<br />] | |||
| | OtherCpds = ]<br/>] | | OtherCpds = ]<br />] | ||
| }} | }} | ||
| }} | }} | ||
Revision as of 12:29, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 475232701 of page Tartaric_acid with values updated to verified values. |
 | |
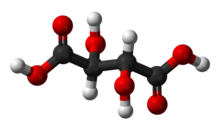 | |
| Names | |
|---|---|
| IUPAC name 2,3-dihydroxybutanedioic acid | |
| Other names
2,3-dihydroxysuccinic acid threaric acid racemic acid uvic acid paratartaric acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | tartaric+acid |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O6 (Basic formula) HO2CCH(OH)CH(OH)CO2H (Structural formula) |
| Molar mass | 150.087 g/mol |
| Appearance | white powder |
| Density | 1.79 g/mL (H2O) |
| Melting point | 171–174 °C (L or D-tartaric; pure) 206 °C (DL, racemic) 165-166°C ("meso-anhyrdous") 146–148 °C (meso-hydrous) |
| Solubility in water | 133 g/100ml (20 °C) |
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.95 pKa2= 4.25 meso 25 °C: pKa1= 3.22 pKa2= 4.85 |
| Related compounds | |
| Other cations | Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
- Tartaric Acid – Compound Summary, PubChem.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.