| Revision as of 13:27, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473591833 of page Erbium(III)_chloride for the Chem/Drugbox validation project (updated: 'CASNo').← Previous edit |
Revision as of 13:27, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 472446794 of page Dithionic_acid for the Chem/Drugbox validation project (updated: 'CASNo').Next edit → |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{chembox |
|
{{Chembox |
|
⚫ |
| verifiedrevid = 450839291 |
|
| Watchedfields = changed |
|
|
|
| ImageFile1 = Dithionic-acid-2D.png |
| ⚫ |
| verifiedrevid = 428984587 |
|
|
|
| ImageFile2 = Dithionic-acid-3D-balls.png |
|
| Name = Erbium(III) chloride |
|
|
|
| ImageAlt2 = Ball-and-stick model of dithionic acid |
|
| ImageFile = Erbium(III)chloride sunlight.jpg |
|
|
|
| IUPACName = dithionic acid <ref>{{RedBookRef|page=130}}</ref> |
|
| ImageName = Erbium(III) chloride hydrate photographed in sunlight |
|
|
|
| OtherNames = hypodisulfuric acid |
|
| IUPACName = Erbium(III) chloride |
|
|
| OtherNames = Erbium trichloride |
|
|
| Section1 = {{Chembox Identifiers |
|
| Section1 = {{Chembox Identifiers |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 59656 |
|
| ChemSpiderID = 25128 |
|
| InChI = 1/3ClH.Er/h3*1H;/q;;;+3/p-3 |
|
| InChI = 1/H2O6S2/c1-7(2,3)8(4,5)6/h(H,1,2,3)(H,4,5,6) |
|
| InChIKey = HDGGAKOVUDZYES-DFZHHIFOAE |
|
| InChIKey = RMGVZKRVHHSUIM-UHFFFAOYAM |
|
| SMILES = Cl(Cl)Cl |
|
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/3ClH.Er/h3*1H;/q;;;+3/p-3 |
|
| StdInChI = 1S/H2O6S2/c1-7(2,3)8(4,5)6/h(H,1,2,3)(H,4,5,6) |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = HDGGAKOVUDZYES-UHFFFAOYSA-K |
|
| StdInChIKey = RMGVZKRVHHSUIM-UHFFFAOYSA-N |
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo = <!-- blanked - oldvalue: 10138-41-7 --> |
|
| CASNo = <!-- blanked - oldvalue: 14970-71-9 --> |
|
⚫ |
| PubChem = 26985 |
|
| CASOther (anhydrous)<br/> (hexahydrate) |
|
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ⚫ |
| PubChem = 66277 |
|
|
|
| ChEBI = 29208 |
| ⚫ |
}} |
|
|
|
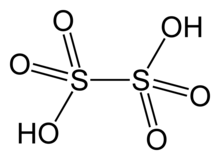
| SMILES = O=S(=O)(O)S(=O)(=O)O |
|
|
}} |
|
| Section2 = {{Chembox Properties |
|
| Section2 = {{Chembox Properties |
|
| Formula = ErCl<sub>3</sub> (anhydrous)<br/>ErCl<sub>3</sub>·6H<sub>2</sub>O (hexahydrate) |
|
| Formula = H<sub>2</sub>S<sub>2</sub>O<sub>6</sub> |
|
| MolarMass = 273.62 g/mol (anhydrous)<br/>381.71 g/mol (hexahydrate) |
|
| MolarMass = 162.14 g mol<sup>−1</sup> |
|
|
| Appearance = |
|
| Appearance = violet ] ] crystals (anhydrous)<br/>pink ] crystals (hexahydrate) |
|
|
| Density = 4.1 g/cm<sup>3</sup> (anhydrous) |
|
| Density = |
|
| MeltingPt = 776 °C (anhydrous)<br/>decomposes (hexahydrate) |
|
| MeltingPt = |
|
| BoilingPt = 1500 °C |
|
| BoilingPt = |
|
|
| Solubility = |
|
| Solubility = soluble in ] (anhydrous)<br/>slightly soluble in ] (hexahydrate)<ref name="hand"> |
|
|
⚫ |
}} |
|
{{Cite book |
|
|
|
| Section3 = {{Chembox Hazards |
|
| last = Lide |
|
|
|
| MainHazards = |
|
| first = David R. |
|
|
| author-link = |
|
| FlashPt = |
|
| last2 = |
|
| Autoignition = |
|
| first2 = |
|
|
| author2-link = |
|
|
| publication-date = |
|
|
| date = |
|
|
| year = 1998 |
|
|
| title = Handbook of Chemistry and Physics |
|
|
| edition = 87 |
|
|
| volume = |
|
|
| series = |
|
|
| publication-place = Boca Raton, FL |
|
|
| place = |
|
|
| publisher = CRC Press |
|
|
| id = |
|
|
| isbn = 0-8493-0594-2 |
|
|
| doi = |
|
|
| oclc = |
|
|
| pages = 4–57 |
|
|
| url = |
|
|
| accessdate = |
|
|
| postscript = <!--None--> |
|
|
}}</ref> |
|
|
}} |
|
}} |
|
}} |
|
}} |