| Revision as of 21:18, 23 May 2013 view sourceBlackHades (talk | contribs)Extended confirmed users1,361 edits Undid revision 556418577 by 125.18.242.18 (talk)← Previous edit | Revision as of 01:26, 26 May 2013 view source Furious Style (talk | contribs)122 edits →HealthNext edit → | ||
| Line 24: | Line 24: | ||
| Governments worldwide assess and manage the risks associated with the ] and the marketing of ]. There are differences in the ] of GM food, and therefore in the regulation of GMOs, between countries. Some of the most marked differences occur between the USA and Europe. Crops not intended for food use are generally not reviewed by authorities responsible for food safety.<ref name=PotatoPro>{{cite web |url=http://www.potatopro.com/newsletters/20100310.htm |work=PotatoPro |first=Paul |last=van Eijck |date=10 March 2010 |title=The History and Future of GM Potatoes}}</ref> Food derived from GMOs is not tested in humans before it is marketed as it is not a single chemical, nor is it intended to be ingested in specific doses and times, which makes it difficult to design meaningful ].<ref name=UC-ANR8180>Winter, CK and Gallegos, LK. 2006. University of California Agricultural and Natural Resource Service. ANR Publication 8180. </ref> Regulators examine the genetic modification, its protein products, and any intended changes that those proteins make to the food.<ref>EFSA Panel on Genetically Modified Organisms (GMO) (2011) ''EFSA Journal'' 9(5) 2150 . </ref> Regulators also check to see whether the food derived from a GMO is "substantially equivalent" to its non-GM-derived counterpart, which provides a way to detect any negative non-intended consequences of the genetic engineering.<ref name=UC-ANR8180 /> If the newly incorporated protein is not similar to that of other proteins found in food or if anomalies arise in the substantial equivalence comparison, further ] testing is required.<ref name=UC-ANR8180 /> | Governments worldwide assess and manage the risks associated with the ] and the marketing of ]. There are differences in the ] of GM food, and therefore in the regulation of GMOs, between countries. Some of the most marked differences occur between the USA and Europe. Crops not intended for food use are generally not reviewed by authorities responsible for food safety.<ref name=PotatoPro>{{cite web |url=http://www.potatopro.com/newsletters/20100310.htm |work=PotatoPro |first=Paul |last=van Eijck |date=10 March 2010 |title=The History and Future of GM Potatoes}}</ref> Food derived from GMOs is not tested in humans before it is marketed as it is not a single chemical, nor is it intended to be ingested in specific doses and times, which makes it difficult to design meaningful ].<ref name=UC-ANR8180>Winter, CK and Gallegos, LK. 2006. University of California Agricultural and Natural Resource Service. ANR Publication 8180. </ref> Regulators examine the genetic modification, its protein products, and any intended changes that those proteins make to the food.<ref>EFSA Panel on Genetically Modified Organisms (GMO) (2011) ''EFSA Journal'' 9(5) 2150 . </ref> Regulators also check to see whether the food derived from a GMO is "substantially equivalent" to its non-GM-derived counterpart, which provides a way to detect any negative non-intended consequences of the genetic engineering.<ref name=UC-ANR8180 /> If the newly incorporated protein is not similar to that of other proteins found in food or if anomalies arise in the substantial equivalence comparison, further ] testing is required.<ref name=UC-ANR8180 /> | ||
| There is broad scientific consensus that food on the market derived from GM crops is safe |
There is broad scientific consensus that food on the market derived from GM crops is safe to eat.<ref name="agbioworld.org"/><ref name="UC-Safety"/><ref name=SciAm /><ref name=OECD2000>Organisation for Economic Co-operation and Development. Report of the Task Force for the Safety of Novel Foods and Feeds C(2000)86/ADD1. 17 May 2000 page 4, paragraph 4.</ref> In 2012, the ] stated "Foods containing ingredients from genetically modified (GM) crops pose no greater risk than the same foods made from crops modified by conventional plant breeding techniques."<ref>AAAS Board of Directors. (2012) </ref> The ], the ] and the ] have stated that no adverse health effects on the human population related to GM food have been reported and/or substantiated in peer-reviewed literature to date.<ref name="NRC2004"/><ref name="Key"/><ref name="Labeling of Bioengineered Foods"/> The European Commission Directorate-General for Research and Innovation 2010 report on GMOs noted that "The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research, and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies."<ref name="decade_of_EU-funded_GMO_research">{{cite book |title= A decade of EU-funded GMO research (2001-2010)|url= http://ec.europa.eu/research/biosociety/pdf/a_decade_of_eu-funded_gmo_research.pdf|format= pdf|year= 2010|publisher= Directorate-General for Research and Innovation. Biotechnologies, Agriculture, Food. European Union|doi= 10.2777/97784|isbn= 978-92-79-16344-9|page= 16}}</ref> A 2004 report by Working Group 1 of the ENTRANSFOOD project, a group of scientists funded by the European Commission to identify prerequisites for introducing agricultural biotechnology products in a way that is largely acceptable to European society,<ref>CORDIS - Community Research and Development Information Service. 2005-01-06 </ref> concluded that "the combination of existing test methods provides a sound test-regime to assess the safety of GM crops."<ref name=Konig>{{cite journal | author = König A, Cockburn A, Crevel RW, Debruyne E, Grafstroem R, Hammerling U, Kimber I, Knudsen I, Kuiper HA, Peijnenburg AA, Penninks AH, Poulsen M, Schauzu M, Wal JM | title = Assessment of the safety of foods derived from genetically modified (GM) crops | journal = Food Chem. Toxicol. | volume = 42 | issue = 7 | pages = 1047–88 | year = 2004 | month = July | pmid = 15123382 | doi = 10.1016/j.fct.2004.02.019 }}</ref> | ||
| ] | ] | ||
Revision as of 01:26, 26 May 2013
For related content, see genetic engineering, genetically modified food, genetically modified crops, and regulation of the release of genetic modified organisms.The genetically modified foods controversy is a dispute over the relative advantages and disadvantages of food derived from genetically modified organisms, genetically modified crops used to produce food and other goods, and other uses of genetically modified organisms in food production. The dispute involves consumers, biotechnology companies, governmental regulators, non-governmental organizations and scientists. The key areas of controversy related to genetically modified (GM) food are: risk of harm from GM food, whether GM food should be labeled, the role of government regulators, the effect of GM crops on the environment, the impact of GM crops for farmers, including farmers in developing countries, the role of GM crops in feeding the growing world population, and GM crops as part of the industrial agriculture system.
There is broad scientific consensus that food on the market derived from GM crops pose no greater risk than conventional food. No reports of ill effects have been documented in the human population from GM food. Supporters of food derived from GMOs hold that food is as safe as other foods and that labels send a message to consumers that GM food is somehow dangerous. They trust that regulators and the regulatory process are sufficiently objective and rigorous, and that risks of contamination of the non-GM food supply and of the environment can be managed. They trust that there is sufficient law and regulation to maintain competition in the market for seeds, believe that GM technology is key to feeding a growing world population, and view GM technology as a continuation of the manipulation of plants that humans have conducted for millennia.
Advocacy groups such as Greenpeace and World Wildlife Fund have concerns that risks of GM food have not been adequately identified and managed, and have questioned the objectivity of regulatory authorities. Opponents of food derived from GMOs are concerned about the safety of the food itself and wish it banned, or at least labeled. They have concerns about the objectivity of regulators and rigor of the regulatory process, about contamination of the non-GM food supply, about effects of GMOs on the environment, about industrial agriculture in general, and about the consolidation of control of the food supply in companies that make and sell GMOs, especially in the developing world. Some are concerned that GM technology tampers too deeply with nature.
Public perception
Social science surveys have documented that individuals are more risk averse about food than institutions. There is widespread concern within the public about the risks of biotechnology, a desire for more information about the risks themselves and a desire for choice in being exposed to risk. The introduction of so-called "wonder-products" such as DDT and PCBs and their subsequent withdrawal after unforeseen problems were discovered, has undermined public trust in companies that introduce products that are pervasively used, and in the government agencies meant to regulate them. There is also a widespread sense that social and technological change is speeding up and people feel powerless to affect this change; diffuse anxiety driven by this context becomes focused when it is food that is being changed.
In 2006, the Pew Initiative on Food and Biotechnology made public a review of U.S. survey results from 2001-2006. The review showed that Americans' knowledge of genetically modified foods and animals was low through the period. During this period there were protests against Calgene's Flavr Savr transgenic tomato that described the GM tomato as being made with fish genes, confusing it with DNA Plant Technology's Fish tomato experimental transgenic organism, which was never commercialized.
A 2010 Deloitte survey found that 34% of U.S. consumers were very or extremely concerned about GM food, a 3% reduction from 2008. The same survey found a strong gender difference in opinion: 10% of men were extremely concerned, compared with 16% of women, and 16% of women were unconcerned, compared with 27% of men. A 2009 review article of European consumer polls concluded that opposition to GMOs in Europe has been gradually decreasing. Approximately half of European consumers accepted gene technology, particularly when benefits for consumers and for the environment could be linked to GMO products. 80% of respondents did not cite the application of GMOs in agriculture as a significant environmental problem. Many consumers seem unafraid of health risks from GMO products and most European consumers did not actively avoid GMO products while shopping. The 2010 "Eurobarometer" survey, which assesses public attitudes about biotech and the life sciences in Europe, found that "cisgenics, GM crops produced by adding only genes from the same species or from plants that are crossable by conventional breeding," evokes a different reaction than transgenic methods, where "genes are taken from other species or bacteria that are taxonomically very different from the gene recipient and transferred into plants." A 2007 survey by the Food Standards Australia and New Zealand found that in Australia where labeling is mandatory, 27% of Australians looked at the label to see if it contained GM material when purchasing a grocery product for the first time.
There is a concerted and organised effort from many environmental and other advocacy groups to impose moratoriums or ban GMO products from being commercialised. International organisations like Greenpeace and Friends of the Earth include genetic engineering as part of their environmental and political concerns. Other groups like GMWatch and The Institute of Science in Society concentrate mostly or solely on opposing genetically modified crops.
Opponents of GM food have been labelled "the Climate Skeptics of the Left" by Keith Kloor of Slate Magazine. Others have labelled the anti-GM ideologies as conspiracy theories.
Health
Governments worldwide assess and manage the risks associated with the release of genetically modified organisms and the marketing of genetically modified food. There are differences in the risk assessment of GM food, and therefore in the regulation of GMOs, between countries. Some of the most marked differences occur between the USA and Europe. Crops not intended for food use are generally not reviewed by authorities responsible for food safety. Food derived from GMOs is not tested in humans before it is marketed as it is not a single chemical, nor is it intended to be ingested in specific doses and times, which makes it difficult to design meaningful clinical studies. Regulators examine the genetic modification, its protein products, and any intended changes that those proteins make to the food. Regulators also check to see whether the food derived from a GMO is "substantially equivalent" to its non-GM-derived counterpart, which provides a way to detect any negative non-intended consequences of the genetic engineering. If the newly incorporated protein is not similar to that of other proteins found in food or if anomalies arise in the substantial equivalence comparison, further toxicological testing is required.
There is broad scientific consensus that food on the market derived from GM crops is safe to eat. In 2012, the American Association for the Advancement of Science stated "Foods containing ingredients from genetically modified (GM) crops pose no greater risk than the same foods made from crops modified by conventional plant breeding techniques." The American Medical Association, the National Academies of Sciences and the Royal Society of Medicine have stated that no adverse health effects on the human population related to GM food have been reported and/or substantiated in peer-reviewed literature to date. The European Commission Directorate-General for Research and Innovation 2010 report on GMOs noted that "The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research, and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies." A 2004 report by Working Group 1 of the ENTRANSFOOD project, a group of scientists funded by the European Commission to identify prerequisites for introducing agricultural biotechnology products in a way that is largely acceptable to European society, concluded that "the combination of existing test methods provides a sound test-regime to assess the safety of GM crops."
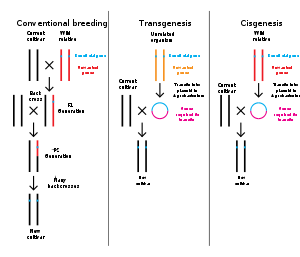
There is a view from many of the scientists and regulators who support GM food that there is a continuing need for improved testing technologies and protocols to identify and manage risk better. A consensus document released by the OECD in 2010 says that molecular characterisation by itself is not the best way to predict the safety of GM plants, but can focus the other safety assessment procedures. They also suggest that new technologies will develop that will aid in the "food, feed and environmental risk/safety assessments. " While generally transgenic and cisgenic organisms are treated similarly when assessed, in 2012 the European Food Safety Authority (EFSA) Panel on Genetically Modified Organisms (GMO) has said that "novel hazards" could be associated with transgenic crops that will not be present in cisgenic ones. Advocacy groups such as Greenpeace, World Wildlife Fund, Organic Consumers Association, and Center for Food Safety have concerns that potential risks to health and the environment relating to GM have not yet been adequately investigated. In Japan, the Consumers Union of Japan say that truly independent research in these areas is systematically blocked by the GM corporations which own the GM seeds and reference materials. Independence in research has been studied by a 2011 analysis into conflicts of interest which found a significant correlation between author affiliation to industry and study outcome in scientific work published on health risks or nutritional assessment studies of genetically modified products.
Substantial equivalence
The starting point for the safety assessment of genetically engineered food products by regulatory bodies is to assess if the food is "substantially equivalent" to their counterparts, which themselves are the products of genetic manipulation via traditional methods of cross-breeding and hybridization. The application of substantial equivalence has been criticized. In 1999, Andrew Chesson of the University of Aberdeen warned that substantial equivalence testing "could be flawed in some cases" and that some current safety tests could allow harmful substances to enter the human food chain. The same year Erik Millstone, Eric Brunner and Sue Mayer argued in a commentary in Nature that the substantial equivalence standard was pseudo-scientific and was the product of politics and business lobbying—they claimed it was created primarily to reassure consumers and to aid biotechnology companies in avoiding the time and cost of more rigorous safety testing. They suggested that all GM foods should have extensive biological, toxicological and immunological tests and that the concept of substantial equivalence should be abandoned. This commentary was criticized for providing a misleading presentation of history, for distorting existing data and applying bad logic. Retired scientist Harry Kuiper said it presented an oversimplified version of safety assessments and that equivalence testing involves more than chemical tests and may include toxicity testing. An opinion piece in the Los Angeles Times in 2001 by Barbara Keeler and Marc Lappe supported legislation in the US Congress to set aside the substantial equivalence standard and instead mandate that safety studies be performed.
This process was examined further in a 2002 review published by Harry Kuiper 2002 in the journal Toxicology. It stated that substantial equivalence does not measure risks, but instead identifies differences between existing products and new foods, which might pose dangers to health. If differences do exist, identifying these differences is a starting point for a full safety assessment, rather than an end point. It concluded that "The concept of substantial equivalence is an adequate tool in order to identify safety issues related to genetically modified products that have a traditional counterpart". The review also noted difficulties in applying this standard in practice, including the fact that traditional foods contain many chemicals that have toxic or carcinogenic effects and that our existing diets therefore have not been proven to be safe. This lack of knowledge on unmodified food poses a problem, as GM foods may have differences in anti-nutrients and natural toxins that have never been identified in the original plant, raising the possibility that harmful changes could be missed. The possibility also exists that positive modifications may be missed. For example, corn damaged by insects often contains high levels of fumonisins, carcinongic toxins made by fungi that are carried on the backs of insects and that grow in the wounds of the damaged corn. Studies show that most Bt corn has lower levels of fumonisins than conventional corn damaged by insects. Regulators are aware of these issues and workshops and consultations organized by the OECD, WHO, and FAO have worked to acquire data and develop standards for conventional foods, for use in assessing substantial equivalence.
A survey of publications describing comparisons between the intrinsic qualities of GM and non-GM reference crop lines (comparing genomes, proteomes, and metabolomes of the plants themselves, not the plants' effects on an organism eating them) indicates that transgenic modification of crops has less impact on gene expression or on protein and metabolite levels than the variability generated by conventional breeding.
In a 2013 review published in the Journal of Agricultural and Food Chemistry, Rod A. Herman (Dow AgroSciences) and William D. Price (retired from FDA) argue that transgenesis is less disruptive of composition compared with traditional breeding techniques which routinely involve genetic mutations, deletions, insertions, and rearrangements. The FDA found all of the 148 transgenic events that they evaluated to be substantially equivalent to their conventional counterparts, as have the Japanese regulators for 189 submissions including combined-trait products. This equivalence is confirmed by over 80 peer-reviewed publications. Hence, the authors argue, compositional equivalence studies uniquely required for GM crops may no longer be justified on the basis of scientific uncertainty.
Allergenicity
Regulatory authorities require that new GM foods be tested for allergenicity before they are marketed.
Some environmental organizations, such as the European Green Party and Greenpeace, have suggested that GM food might trigger food allergies. A 2005 review in the journal Allergy of the results from allergen testing of current GM foods stated that "no biotech proteins in foods have been documented to cause allergic reactions".
The development of GM products which have been found to cause allergic reactions have been halted by the companies developing them before they were brought to market. In the early 1990s, Pioneer Hi-Bred attempted to improve the nutrition content of soybeans intended for animal feed by adding a gene from the Brazil nut. Because they knew that people have allergies to nuts, Pioneer ran both in vitro tests for allergy, in which they tested whether serum from people with nut allergies reacted to the transgenic soy; they also did skin prick tests with protein from the transgenic soy. The tests showed that the transgenic soy was allergenic. Pioneer Hi-Bred therefore discontinued further development. In 2005, a pest-resistant field pea developed by the Australian Commonwealth Scientific and Industrial Research Organisation for use as a pasture crop was shown to cause an allergic reaction in mice. Work on this variety was immediately halted. These cases of products that failed safety testing have been viewed as evidence that genetic modification can produce unexpected and dangerous changes in foods, and as evidence that the current tests are effective at identifying safety problems before foods come on the market.
Genetic modification can also be used to remove allergens from foods, potentially reducing the risk of food allergies. A hypo-allergenic strain of soybean was tested in 2003 and shown to lack the major allergen that is found in the beans. A similar approach has been tried in ryegrass, which produces pollen that is a major cause of hay fever: here a fertile GM grass was produced that lacked the main pollen allergen, demonstrating that the production of hypoallergenic grass is also possible.
Horizontal gene transfer from plants to animals
One concern raised has been the possibility of the transgenes transferring from plants used as feed to animals that are used for food, or from plants as used as food, to humans. See below for environmental risks of gene flow among plants.
The risk of horizontal gene transfer between plants and animals is very low and in most cases with GM crops this is expected to be lower than background rates. Two studies on the possible effects of giving genetically modified feed to animals found no residues of recombinant DNA or novel proteins have been found in any organ or tissue samples obtained from animals fed with GMP plants. Studies have found DNA from the M13 virus, Green fluorescent protein and Rubisco genes in the blood and tissue of animals and in 2012 a paper showed that a specific microRNA from rice could be found at very low quantities in human and animal serum.
Of particular concern is that the antibiotic resistance gene commonly used as a genetic marker in transgenic crops could be transferred to harmful bacteria, creating superbugs that are resistant to multiple antibiotics. In 2004 a study involving human volunteers was conducted to see if the transgene from GM soy would transfer to the bacteria that naturally lives in the human gut. As of 2012 it is the only human feeding study conducted with genetically modified food. The transgene was only detected in three volunteers, part of seven who had previously had their large intestines removed for medical reasons. As this gene transfer did not increase after the consumption of GM soy, the researchers concluded that gene transfer did not occur during the experiment. In volunteers with complete digestive tracts, the transgene did not survive passage through intact gastrointestinal tract. The antibiotic genes used in genetic engineering are already found in many natural pathogens, commonly used during animal husbandry and not widely used prescribed.
Animal feeding studies
A 2012 review of more than 24 long-term animal feeding studies conducted by public research laboratories, concluded that none of these studies discovered any safety problem linked to long-term consumption of GM food. A 2009 review by Magaña-Gómez et al. found that although most studies concluded that GM foods do not differ in nutrition or cause any detectable toxic effects in animals, some studies did report adverse changes at a cellular level caused by some GM foods, concluding that "More scientific effort and investigation is needed to ensure that consumption of GM foods is not likely to provoke any form of health problem". A review published in 2009 by Dona and Arvanitoyannis concluded that "results of most studies with GM foods indicate that they may cause some common toxic effects such as hepatic, pancreatic, renal, or reproductive effects and may alter the hematological, biochemical, and immunologic parameters". However responses to this review in 2009 and 2010 note that the Dona and Arvanitoyannis concentrated on articles with an anti-GM bias that have been refuted by scientists in peer-reviewed articles elsewhere - for example the 35S promoter, stability of transgenes, antibiotic marker genes and the claims for toxic effects of GM foods. Gerhard Flachowsky concluded in a 2005 review that the current GM food with only a single gene modification are similar in nutrition and safety to non-GM foods, but noted that food with multiple gene modifications would be more difficult to test, and would require further animal studies. A 2004 review of animal feeding trials by Aumaitre et al. found no differences among animals eating genetically modified plants.
In 2007, José L. Domingo searched the Pubmed database using 12 search terms and found 68 papers showing animal studies involving GM food. He concluded that the "number of references" on the safety of GM/transgenic crops was "surprisingly limited" and questioned whether the safety of genetically modified food has been demonstrated; the review also remarked that its conclusions were in agreement with three earlier reviews. In contrast, Philippe Vain found 692 research studies in 2007 that focused on GM crop and food safety and identified a strong increase in the publication of such articles in recent years. Vain commented that the multidisciplinarian nature of GM research complicates the retrieval of GM studies and requires using many search terms (he used more than 300) and multiple databases. Domingo again reviewed the literature in 2011 and said that although there had been a substantial increase in the number of studies since 2006, most were conducted by the biotechnology companies responsible for commercialising the plants.
Human studies and obstacles
While some groups and individuals have called for more human testing of GM food, there are several obstacles to such studies. Both the US General Accounting Office (in a review of FDA procedures requested by Congress) and the FAO/WHO have confirmed that long term studies of the effect of GM food on humans are not feasible, for reasons including: there is no plausible hypothesis to test; very little is known about the potential long-term effects of any foods; identification of such effects is further confounded by the great variability in the way people react to foods; and epidemiological studies are not likely to differentiate the health effects of GM foods from the many undesirable effects of conventional foods. Additionally, there are strong ethics that guide the conduct of research on human subjects, which mandate that the intervention being tested must have a potential benefit for the human subjects, such as treatment for a disease or nutritional benefit (ruling out toxicity testing on humans). In this context, scientists and regulators discussing clinical studies of GM food have written that the "ethical and technical constraints of conducting human trials, and the necessity of doing so, is a subject that requires considerable attention." Golden rice has been tested in humans to see if the rice provides a nutritional benefit, namely, increased levels of Vitamin A. However the authors of a study in Chinese children published in 2012 have come under fire for not obtaining the consent of the parents of the children nor the Chinese government.
Individual studies
There have been some individual studies published in journals that have suggested negative impacts from eating GM food. The first such peer reviewed paper to be published was in 1999 and covered research conducted by Arpad Pusztai in 1998. Pusztai had fed rats GM potatoes transformed with the Galanthus nivalis agglutinin (GNA) gene from the Galanthus (snowdrop) plant, allowing the GNA lectin protein to be synthesised. Lectin is known to be toxic, especially to gut epithelium, and while some companies were considering making GM crops expressing lectin, GNA was an unlikely candidate. On June 22, 1998 a short interview was shown on Granada Television's current affairs programme World in Action, with Pusztai saying that rats fed the potatoes had stunted growth and a repressed immune system. A media frenzy resulted and Pusztai was suspended from the Rowett Institute with misconduct procedures used to seize his data and ban him from speaking publicly. The Rowett Institute and the Royal Society reviewed Pusztai's work and concluded that the data did not support his conclusions. When his work was eventually published in The Lancet it reported significant differences in the thickness of the gut epithelium of rats fed genetically modified potatoes (compared to those fed the control diet), but no differences in growth or immune system function were suggested. The published paper was criticised on the grounds that the unmodified potatoes were not a fair control diet, and that any rats fed only on potatoes will suffer from a protein deficiency. Pusztai responded to these criticisms by stating that all the diets had the same protein and energy content and that the food intake of all rats was the same. The incident became known as the Pusztai affair.
In 2007, 2009, and 2011 Gilles-Eric Seralini published re-analysis studies that used data from Monsanto rat feeding experiments for three GM maize varieties (insect resistant MON 863 and MON 810, and the glyphosate resistance NK603). He concluded that they had actually caused liver, kidney, and heart damage in the rats. The European Food Safety Authority (EFSA) reviewed the data and concluded that the small differences were all within the normal range for control rats. The EFSA review also stated that the statistical methods used were incorrect. The EFSA conclusions were supported by Food Standards Australia New Zealand (FSANZ), a panel of toxicologists funded by Monsanto and the French High Council of Biotechnologies Scientific Committee (HCB).
In 2012 the Séralini lab published a paper that looked at the long term effects of feeding rats various levels of GM roundup resistance maize, maize spiked with the roundup chemical and a mixture of the two. The paper concluded that rats fed GM maize had an increased incidence of cancer. Once published, there was widespread criticism of the study. Séralini held a press conference just before the paper was released; he allowed reporters access to the paper before his press conference only if they signed a confidentiality agreement under which they could not get other scientists' responses to the paper. This method of announcing the research met with strong criticism from scientists and some journalists as it excluded critical commentary in the breaking stories. Many claimed that Séralini's conclusions were impossible to justify given the statistical power of the study and that Sprague-Dawley rats were not appropriate for a lifetime study (as opposed to a shorter toxicity study) because these rats have a high tendency to get cancer over their lifespan (one study found over 80% got cancer under normal conditions). For a similar study the Organisation for Economic Co-operation and Development guidelines recommend using 65 rats per experiment, not 10. Questions were also raised about the statistical method chosen to analyse the data and the lack of data regarding the amount of food fed to the rats and their growth rates. Other criticisms included the lack of a dose–response relationship (females fed three times the dose showed a decreased number of tumours) and no identifiable mechanism for the increase in tumours. Six French national academies of science issued an unprecedented joint statement condemning the study and the journal that published it. Food and Chemical Toxicology published 17 letters to the editor that expressed strong criticism of the Seralini paper. National food safety and regulatory agencies also reviewed the paper and dismissed it. In March 2013, Seralini responded to these criticisms in the same journal that originally published his study.
A 2011 study, the first to evaluate the correlation between maternal and fetal exposure to Bt toxin produced in genetically modified maize and to determine exposure levels of the pesticides and their metabolites, reported the presence of pesticides associated with GM foods in both non-pregnant women and pregnant women and their fetuses. The paper and the media reports around it were criticized for overstating the results. FSANZ took the unusual step of posting a direct response, saying that the suitability of the ELISA assay method for detecting the Cry1Ab protein was not validated and that there was no evidence that that GM food was the source of the protein. They also suggested that even if the protein was detected it was more likely to come from conventional or organic sources.
Environment
Genetically modified crops are planted in fields much like regular crops. There they interact directly with organisms that feed on the crops, and indirectly with other organisms in the wider food chain. The pollen from the plants behaves like the pollen of any other crop. This has led to concerns about effects of genetically-engineered crops on non-target species, and about gene flow to other plants, animals and bacteria. Some supporters of GM crops see these crops as providing benefits to the environment through a reduction in the use of pesticides and a reduction in greenhouse gas emissions.
Non target organisms
One of the major uses of GM crops is in insect pest control though the expression of the cry (crystal delta-endotoxins) and cyt (cytolysins) genes from Bacillus thuringiensis (Bt). There are concerns that these toxins could target predatory and other beneficial or harmless insects as well as the targeted pest insect. The proteins produced by Bt have been used as organic sprays for insect control in France since 1938 and the USA since 1958 with no ill effects on the environment reported. While cyt proteins are toxic towards the insect orders Coleoptera (beetles) and Diptera (flies), cry proteins selectively target Lepidopterans (moths and butterflies). As a toxic mechanism, cry proteins bind to specific receptors on the membranes of mid-gut (epithelial) cells resulting in rupture of those cells. Any organism that lacks the appropriate receptors in its gut cannot be affected by the cry protein, and therefore Bt. Regulatory agencies assess the potential for the transgenic plant to impact non target organisms before approving their commercial release.
In 1999 a paper was published in Nature showing that in a lab environment pollen from Bt maize dusted onto milkweed could harm the monarch butterfly. A collaborative research exercise was carried out over the next two years by several groups of scientists in the US and Canada, looking at the effects of Bt pollen in both the field and the laboratory. This resulted in a risk assessment that concluded that any risk posed by the corn to butterfly populations under real-world conditions was negligible. A 2002 review of the scientific literature concluded that "the commercial large-scale cultivation of current Bt–maize hybrids did not pose a significant risk to the monarch population" and noted that despite large-scale planting of GM crops, the butterfly's population is increasing.
An analysis of laboratory settings found that Bt toxins can affect nontarget organisms, usually organisms closely related to the intended targets. Typically, exposure occurs through the consumption of plant parts, such as pollen or plant debris, or through Bt ingestion by their predatory food choices. The methodology used by Lövei et al. has been called into question by a group of academic scientists who wrote "We are deeply concerned about the inappropriate methods used in their paper, the lack of ecological context, and the authors’ advocacy of how laboratory studies on non-target arthropods should be conducted and interpreted".
Biodiversity
There are concerns that the genetic diversity of various crops will decrease (as the development of GM varieties will lead to less cultivars being used overall) or that they will indirectly affect the diversity of other organisms. Also, there are concerns that the widespread use of GM crops designed to resist agrochemicals, leads to increased use of those agrochemicals, which in turn causes damage to the environment and to biodiversity.
Studies comparing the genetic diversity of cotton have found that in the USA the diversity has either increased or stayed the same, while in India it has reduced. This has been put down to the larger number of breeding varieties the technology was used on in the USA compared to India. A review of the effects of Bt crops on soil ecosystems found that in general they "appear to have no consistent, significant, and long-term effects on the microbiota and their activities in soil". The diversity and number of weed populations has been shown to decrease in farm-scale trials in the UK and Denmark when comparing herbicide resistant crops to their conventional counterparts. The UK trial suggested that the diversity of birds could be impacted by the decrease in weed seeds available for feeding. Published data from farms involved in the trials showed that seed eating birds were more abundant on conventional maize after the application of the herbicide, but that there were no significant differences in any other crop or prior to herbicide treatment. A 2012 study found a correlation between the reduction of milkweed in farms that grew glyphosate-resistant crops and the decline in adult monarch butterfly populations in Mexico. The New York Times reported that the study "raises the somewhat radical notion that perhaps weeds on farms should be protected.
A scientific study published in 2005 designed to "simulate the impact of a direct overspray on a wetland" with four different agrochemicals (carbaryl (Sevin), malathion, 2,4-Dichlorophenoxyacetic acid, and glyphosate in a Roundup formulation) by creating artificial ecosystems in tanks and then applying "each chemical at the manufacturer's maximum recommended application rates", found that "species richness was reduced by 15% with Sevin, 30% with malathion, and 22% with Roundup, whereas 2,4-D had no effect". The study has been used by environmental groups to argue that use of agrochemicals causes unintended harm to the environment and to biodiversity.
Emergence of secondary pests
Several studies have documented surges in secondary pests (which are not affected by Bt toxins) within a few years of adoption of Bt cotton. In China, the main problem has been with mirids, which have in some cases "completely eroded all benefits from Bt cotton cultivation". A 2009 study in China concluded that the increase in secondary pests depended on local temperature and rainfall conditions and increased in half the villages studied. The increase in insecticide use for the control of these secondary insects was far smaller than the reduction in total insecticide use due to Bt cotton adoption. Another study published in 2011 was based on a survey of 1,000 randomly selected farm households in five provinces in China and found that the reduction in pesticide use in Bt cotton cultivars is significantly lower than that reported in research elsewhere, consistent with the hypothesis suggested by recent studies that more pesticide sprayings are needed over time to control emerging secondary pests, such as aphids, spider mites, and lygus bugs. Similar problems have been reported in India, with both mealy bugs and aphids.
Gene flow
Genes from a genetically modified organism may pass to another organism just like an endogenous gene. The process is known as outcrossing and can occur in any new open-pollinated crop variety, with newly introduced traits potentially crossing into neighboring crop plants of the same or sometimes closely related species. There are concerns that the spread of genes from modified organisms to unmodified relatives could produce species of weeds resistant to herbicides or could disrupt the ecosystem. This is primarily a concern if the transgenic organism has a significant survival capacity and can increase in frequency and persist in natural populations.
In most countries environmental studies are required prior to the approval of a GM plant for commercial purposes, and a monitoring plan must be presented to identify potential effects which have not been anticipated prior to the approval. In 2009 the government of Mexico created a regulatory pathway for approval of genetically modified maize, but because Mexico is the center of diversity for maize, concerns have been raised about the effect that genetically modified maize could have on local strains. A 2001 report in Nature presented evidence that Bt maize was cross-breeding with unmodified maize in Mexico, although the data in this paper was later described as originating from an artifact and Nature stated that "the evidence available is not sufficient to justify the publication of the original paper". A subsequent large-scale study, in 2005, failed to find any evidence of contamination in Oaxaca. However, other authors have stated that they also found evidence of cross-breeding between natural maize and transgenic maize.
As one of the major genetically modified traits is resistance to herbicides there have been concerns raised about the development of "super weeds".
In 2005, scientists at the UK Centre for Ecology and Hydrology reported the first evidence of horizontal gene transfer of pesticide resistance to weeds, in a few plants from a single season; they found no evidence that any of the hybrids had survived in subsequent seasons.
A study published in 2010 by scientists at the University of Arkansas, North Dakota State University, California State University and the US Environmental Protection Agency showed that about 83 percent of wild or weedy canola tested contained genetically modified herbicide resistance genes. According to the researchers, the lack of reports in the US suggests inadequate oversight and monitoring protocols are in place in the US. The development of weeds resistant to glyphosate, the most commonly applied herbicide, could mean that farmers must return to more labour intensive methods to control weeds, use more dangerous herbicides or till the soil (so increasing then risk of erosion). A 2010 report by the National Academy of Sciences stated that the advent of glyphosate-herbicide resistant weeds could cause the genetically engineered crops to lose their effectiveness unless farmers also use other established weed management strategies.
Another problem is that the gene can get into conventional or organic crops, affecting the trade of these crops as GE-free. Most countries have co-exixtence regulations to try and prevent cross-contamination of GM and non-GM crops. There have also been cases where genes from unapproved crops have escaped. In 2006, American exports of rice to Europe were interrupted when much of the US crop was confirmed to be contaminated with unapproved engineered genes. An investigation by the USDA’s Animal and Plant Health Inspection Service (APHIS) was unable to determine the cause of the contamination. In 2007, the U.S. Department of Agriculture fined Scotts Miracle-Gro $500,000 when modified genetic material from creeping bentgrass, a new golf-course grass Scotts had been testing, was found within close relatives of the same genus (Agrostis) as well as in native grasses up to 21 km (13 mi) away from the test sites, released when freshly cut grass was blown by the wind.
One means that has been explored to avoid environmental contamination is Genetic use restriction technology, also dubbed 'Terminator'. This uncommercialized technology would allow the production of crops with sterile seeds, which would prevent the escape of genetically modified traits. Groups concerned with control of the food supply had expressed concern that the technology would be used to limit access to viable seeds. Another similar hypothetical trait-specific technology known as 'Traitor' or 'T-GURT', requires application of a chemical to genetically modified crops to reactivate engineered traits. These technologies have also caused controversy, as there are fears the technology itself, and the patents on them, would allow companies to further control the market for seeds.
Chemical use
Herbicides
The development of glyphosphate resistant plants has changed the herbicide use profile away from the use of more persistant herbicides with higher toxicity, such as atrazine, metribuzin, and alachlor, which reduced the dangers of herbicide runoff into drinking water. However, a study published in Environmental Sciences Europe by Chuck Benbrook concluded that "...Monsanto's Roundup Ready technology, which dominates corn, soy, and cotton farming, has called forth a veritable monsoon of herbicides, both in terms of higher application rates for Roundup, and, in recent years, growing use of other, more-toxic herbicides."
Pesticides
One of the major environmental benefits from using GM crops is the reduction in the use of pesticides. Insect-resistant Bt-expressing crops will reduce the number of pest insects feeding on these plants without the farmers having to apply as much insecticides. A study published by the UK consultancy PG Economics, concluded that globally pesticide spraying was reduced by 286,000 tons in 2006, decreasing the environmental impact of herbicides and pesticides by 15%. A survey of small Indian farms between 2002 and 2008 concluded that Bt cotton adoption has led to higher yields and lower pesticide use. One study concluded insecticide use on cotton and corn during the years 1996 to 2005 fell by 35.6 million kg of insecticide active ingredient, which is roughly equal to the amount of pesticide applied to arable crops in the EU in one year. A study on the effects of using Bt cotton in six northern provinces of China from 1990 to 2010 concluded that Bt cotton halved the use of pesticides and doubled the level of ladybirds, lacewings and spiders, with the environmental benefits extended to neighbouring crops of maize, peanuts and soybeans.
Resistant insect pests
Resistance evolves naturally after a population has been subjected to intense selection pressure in the form of repeated use of a single herbicide or insecticide. In November 2009, Monsanto scientists found the pink bollworm had become resistant to the first generation Bt cotton in parts of Gujarat, India - that generation expresses one Bt gene, Cry1Ac. This was the first instance of Bt resistance confirmed by Monsanto anywhere in the world. Bollworm resistance to first generation Bt cotton has also been identified in the Australia, China, Spain and the United States. The strategy to delay the emergance of Bt resistant pests has been to have non-GM refuges within the GM crops to dilute any resistant genes that may arise or more recently to develop GM crops that have multiple Bt genes that target different receptors within the insect.
Economics
The economic value derived from growing genetically modified food has been a major selling point for the technology. One of the key reasons for the widespread adoption is the perceived economic benefit the technology brings to farmers, including those in developing nations. A 2010 study by US scientists, found that the economic benefit of Bt corn to farmers in five mid-west states was $6.9 billion over the previous 14 years. They were surprised that the majority ($4.3 billion) of the benefit accrued to non-Bt corn. This was speculated to be because the European Corn Borers that attack the Bt corn die and there are fewer left to attack the non-GM corn nearby. Agriculture economists have calculated that "world surplus $240.3 million for 1996. Of this total, the largest share (59%) went to U.S. farmers. The gene developer, Monsanto, received the next largest share (21%), followed by U.S. consumers (9%), the rest of the world (6%), and the germplasm supplier, Delta and Pine Land Company (5%)." A comprehensive 2012 study by PG Economics, a UK company, concluded that GM crops increased farm incomes worldwide by $14 billion in 2010, with over half this total going to farmers in developing countries.
Claims of major benefits to farmers, including poor farmers in developing countries, have been challenged by opponents. The task of isolating impacts of the technology is complicated by the prevalence of biased observers, and by the rarity of controlled comparisons (such as identical seeds, differing only in the presence or absence of the Bt trait, being grown in identical situations). The main Bt crop being grown by small farmers in developing countries is cotton, and a 2006 exhaustive review of findings on Bt cotton by agricultural economists concluded, "the overall balance sheet, though promising, is mixed. Economic returns are highly variable over years, farm type, and geographical location". Mark Lynas, an environmental activist, believes that an outright rejection of the technology is "illogical and potentially harmful to the interests of poorer peoples and the environment".
Impoverished nations
The effect that genetically modified food may have on developing nations is debated. There is agreement that there is a food supply issue, although there is disagreement on the best ways to solve this. Some scientists suggest that a second Green Revolution with increased use of GM crops is needed to meet the demand for food in the developing world. Others say that there is more than enough food in the world and that the hunger crisis is caused by problems in food distribution and politics, not production. The potential for genetically modified food to help impoverished nations was recognised by the International Assessment of Agricultural Science and Technology for Development, but as of 2008 they found no conclusive evidence that they have offered a solution.
Constraints to the deployment of this technology to impoverished nations are the lack of easy access, expense of modern agricultural equipment, and that certain aspects of the system revolving around intellectual property rights are unfair to "undeveloped countries". Consultative Group on International Agricultural Research (CGIAR), an aid and research organization, was praised by the World Bank for its efforts but suggested they shift to genetics research and productivity enhancement. This plan has several obstacles such as patents, commercial licenses, and the difficulty that third world countries have in accessing the international collection of genetic resources and other intellectual property rights that would educate them about modern technology. The International Treaty on Plant Genetic Resources for Food and Agriculture has attempted to remedy this problem, but results have been inconsistent. As a result, "orphan crops", such as teff, millets, cowpeas, and indigenous plants, which are important in these countries receive little investment.
Yield
There is also debate over whether the use of genetically modified crops increases or decreases yield. The currently commercialised varieties have traits that reduce yield loss from insect pressure or weed interference. There are however crops and animals in being developed with traits aimed at directly increasing the yield, with the closest to commercialisation being salmon with an added growth hormone gene.
A 2010 article supported by CropLife International summarised the results of 49 peer reviewed studies on GM crops worldwide. On average, farmers in developed countries experienced increase in yield of 6% and in underdeveloped countries of 29%. Tillage was decreased by 25–58% on herbicide resistant soybeans, insecticide applications on Bt crops were reduced by 14–76% and 72% of farmers worldwide experienced positive economic results. Another yield gain can be seen with the planting of glyphosate-resistant crops. It allowed farmers to plant rows closer together as they did not have to control post-emergent weeds with mechanical tillage.
Critics of genetic engineered crops disagree that they result in increased yield. In 2009 the Union of Concerned Scientists summarized peer-reviewed studies on the yield contribution of genetic engineered crops—soybeans and maize in the United States. The report concluded that in the United States, other agricultural methods have made a greater contribution to national crop yield increases in recent years than genetic engineering.
Intellectual property
Traditionally, farmers in all nations saved their own seed from year to year. However since the early 1900s hybrid crops have been widely used in the developed world and seeds to grow these crops must be purchased each year from seed producers. The offspring of the hybrid corn, while still viable, lose the beneficial traits of the parents, resulting in the loss of hybrid vigor. In these cases, the use of hybrid plants has been the primary reason for growers not saving seed, not intellectual property issues. However, for non-hybrid biotech crops, such as transgenic soybeans, seed companies use intellectual property law and tangible property common law, each expressed in contracts, to forbid farmers from saving seed. For example, Monsanto's typical bailment license (covering transfer of the seeds themselves) forbids saving seeds, and also requires that purchasers sign a separate patent license agreement.
Corporations say that they need product control in order to prevent seed piracy, to fulfill financial obligations to shareholders, and to invest in further GM development. DuPont spent approximately half its $2 billion R&D budget on agriculture in 2011 while Monsanto spends 9-10% of their sales in their research and development effort every year.
Detractors such as Greenpeace say that patent rights give corporations a dangerous amount of control over their product. Others claim that "patenting seeds gives companies excessive power over something that is vital for everyone." Regarding the issues of intellectual property and patent law, an international report from the year 2000 states: "If the rights to these tools are strongly and universally enforced - and not extensively licensed or provided pro bono in the developing world - then the potential applications of GM technologies described previously are unlikely to benefit the less developed nations of the world for a long time (i.e. until after the restrictions conveyed by these rights have expired).
Monsanto has filed patent infringement suits against 145 farmers, but has proceeded to trial with only 11. Although in some of those 11 cases, a defense of unintentional contamination by gene flow was used, Monsanto won all 11 cases. Monsanto Canada's Director of Public Affairs has stated that "It is not, nor has it ever been Monsanto Canada's policy to enforce its patent on Roundup Ready crops when they are present on a farmer's field by accident...Only when there has been a knowing and deliberate violation of its patent rights will Monsanto act."
One example of such litigation is the Monsanto v. Schmeiser case. This case is widely misunderstood: "The fear about a company claiming ownership of a farmer’s crop based on the inadvertent presence of GM pollen grain or seed is...widespread and ...unfounded." In 1997, Percy Schmeiser, a canola breeder and grower in Bruno, Saskatchewan, discovered that a section of one of his fields contained canola that was resistant to herbicide Roundup by spraying it with Roundup, leaving only the resistant plants. He had not purchased roundup-resistant canola; it was apparently sown from seed blown onto his land from neighboring fields. He later harvested and saved the seed from this area, and replanted the saved seed in 1998. During the 1998 growing season, Monsanto approached Schmeiser and asked him to take a license to the patent covering the transgenic seed he had planted; Schmeiser refused, claiming that he owned the physical seeds he had harvested in 1997 and had the right to do with them as he wished. Monsanto sued Schmeiser for patent infringement and prevailed in the initial case. Schmeiser appealed and lost, and appealed again to the Canadian Supreme Court, which in 2004 ruled 5 to 4 in Monsanto’s favor.
Market dynamics
The seed industry is dominated by several seed and biotechnology firms. Firms have engaged in vertical integration, causing structural changes in the seed industry. It is reported that in 2011, 73% of the global market is controlled by 10 companies.
In 2001, the USDA published an article showing that the concentration of market power in the seed industry has led to economies of scale that facilitated market efficiency because production costs have decreased, however, the move by some companies to divest their seed operations calls into question the long-term viability of these conglomerates. Two economists, guest speakers on the AgBio Forum cite that the huge market power possessed by the small number of biotechnology companies in crop biotechnology could be beneficial in raising welfare despite the pricing strategies they practice because "even though price discrimination is often considered to be an unwanted market distortion, it may increase total welfare by increasing total output and by making goods available to markets where they would not appear otherwise."
Market power gives seed and biotechnology firms the ability to set or influence price, dictate terms, and act as a barrier to entry into the industry. It also gives firms the bargaining power over governments in policy making. In March 2010, the US Justice Department and the U.S. Department of Agriculture held a meeting in Ankeny, Iowa to look at the competitive dynamics in the seed industry. Christine Varney, who heads the antitrust division in the Justice Department, said that her team was investigating whether biotech-seed patents are being abused to extend or maintain companies’ dominance in the industry. A key issue is how Monsanto sells and licenses its patented trait that allows farmers to kill weeds with Roundup herbicide while leaving crops unharmed - the gene was in 93 percent of U.S. soybeans grown in 2009. About 250 family farmers, consumers and other critics of corporate agriculture held a town meeting prior to the governmental meeting to protest Monsanto for what they see as manipulation of the market by buying up independent seed companies, patenting the seeds and then raising seed prices.
International Trade
Europe
GM food and GM crops have been the subject of international trade disputes. Such a dispute arose between the US and Europe in the early 2000s. Until the 1990s, Europe's regulation was less strict than in the United States. In 1998, the use of MON810, a Bt expressing maize conferring resistance to the European corn borer, was approved for commercial cultivation in Europe. However, in the 1990s, a series of unrelated food crises created consumer apprehension about food safety in general and eroded public trust in government oversight of the food industry - most importantly, the infection of cows with bovine spongiform encephalopathy and the mishandling of food safety by European authorities. In 1998, a de facto moratorium led to the suspension of approvals of new genetically modified organisms (GMO) in the European Union pending the adoption of revised rules to govern the approval, marketing and labelling of biotech products.
The approval of GM crops in the US in the mid-1990s precipitated strong public concern in Europe and led to a dramatic decrease in US exports to the EU. "Prior to 1997, corn exports to Europe represented about 4% of total U.S. corn exports, generating about $300 million in sales. Starting in 1997, however, the U.S. largely stopped shipping bulk commodity corn to the EU because such shipments typically commingled corn from many farms, including genetically modified varieties not approved by the EU. The change was dramatic. For example, before 1997, the U.S. sold about 1.75 million tons of corn annually to Spain and Portugal, the two largest importers of U.S. corn in the EU. But in the 1998–99 crop year, Spain bought less than a tenth of the previous year’s amount and Portugal bought none at all."
In May 2003, the United States and twelve other countries filed a formal complaint with the World Trade Organization that the European Union was violating international trade agreements, in blocking imports of U.S. farm products through its long-standing ban on genetically modified food. The countries argued that the EU's regulatory process was far too slow and its standards were unreasonable given the overwhelming body of scientific evidence showing that the crops were safe. The case was also lobbied by U.S. biotechnology giant Monsanto and France's Aventis, as well as by US agricultural groups such as the National Corn Growers Association. In response, in June 2003, the European Parliament ratified a U.N. biosafety protocol regulating international trade in genetically modified food, and in July agreed to new regulations requiring labeling and traceability, as well as an opt-out provision for individual countries. Following this, the approval of new GMOs began again in May 2004. While a number of other GMOs have been approved since then, approvals remain controversial and various countries have utilized the opt-out provisions. In 2006, the WTO ruled that the pre-2004 restrictions had been violations, although the ruling had little immediate effect since the moratorium had already been lifted.
In late 2007, the U.S. ambassador to France recommended "moving to retaliation" to cause "some pain" against France and the European Union in an attempt to fight the French ban and changes in European policy toward genetically modified crops, according to a U.S. government diplomatic cable obtained by WikiLeaks.
Regulation
Labeling

While some groups advocate the complete prohibition of GMOs, others call for mandatory labeling of genetically modified food or other products, while others call for no labeling of GM food.
The European Union, Australia, New Zealand, China, India and other countries require GMO labeling, while others make GMO labeling voluntary or have plans to introduce labeling.
Biotechnology labelling is not required in the United States, although there have been numerous efforts to pass labeling laws. One of the first efforts was on the 2002 Oregon Ballot, which failed to pass by a ratio of 7 to 3. Eighteen state legislatures that debated GM labeling legislation in early 2012 and Vermont's House Agriculture Committee drafted and passed a bill requiring labeling in April 2012, but it was introduced too late in the legislative season to be passed into law during 2012. In 2012, the U.S. state of California voted against Proposition 37, which would have required the labelling of genetically modified food.
The American Medical Association (AMA) and the American Association for the Advancement of Science (AAAS) oppose mandatory labeling of GM food because there is no scientific evidence of harm. The AMA believes that even voluntary labeling is misleading unless accompanied by focused consumer education. The AAAS argues that mandatory labeling "can only serve to mislead and falsely alarm consumers".
A 2007 study on the effect of labeling laws found that once labeling went into effect, few products contained genetically modified ingredients. Businesses stopped carrying products with GM food. The study also found that costs are higher in food-exporting countries than in food-importing countries. Food exporting countries such as the U.S., Argentina, and Canada have adopted voluntary labeling approaches while countries that have adopted mandatory labeling are generally importers of genetically modified food.
A website posted by P. Bryne of the Colorado State University Extension, provides a concise list of pros and cons of labeling food derived from GMOs, with further detail. The list of pros and cons is reproduced here with modifications:
- Pros and Cons of Mandatory Labeling
- There are several arguments put forward in favor of and against mandatory labeling of GM foods. Those arguments are summarized below.
- Pro-mandatory labeling Arguments
- Consumers have a right to know what’s in their food, especially concerning products for which health and environmental concerns have been raised.
- Proponents of mandatory labeling in the US argue that Europe, Japan, India and China require mandatory labeling and that a majority of Americans support mandatory labeling.
- Anti-mandatory labeling Arguments
- Labels on GM food imply a warning about health effects, whereas no significant differences between GM and conventional foods have been detected. If a nutritional or allergenic difference were found in a GM food, current FDA regulations require a label to that effect.
- Labeling of GM foods to fulfill the desires of some consumers would impose a cost on all consumers. Experience with mandatory labeling in the European Union, Japan, and New Zealand has not resulted in consumer choice. Rather, retailers have eliminated GM products from their shelves due to perceived consumer aversion to GM products.
- The Right to know approach (as opposed to the need to know approach) is too open ended and potentially unbounded, because it can be invoked for virtually anything.
- Consumers who want to buy non-GM food already have an option: to purchase certified organic foods that are labelled "100% Organic," which by definition cannot be produced with non-organic ingredients.
- Segregation, identity preservation and systematic testing are costly activities. The providers of the non-GM product have the best incentives to undertake such activities effectively. Therefore, voluntary labelling of the non-GM attribute is preferable from an economics perspective.
Objectivity of regulatory bodies
Groups opposing the release of genetically modified organisms or their use as food have questioned whether regulatory authorities in various countries are too close to companies that seek approval for their products, or have received bribes from such companies.
Critics in the US have protested in regards to the appointment of pro GM lobbyists to senior positions in the FDA. Michael R. Taylor, a former Monsanto lobbyist, was appointed as a senior adviser to the FDA on food safety in 1991. Following his tenure at the FDA, Taylor became a vice-president of Monsanto. On 7 July 2009, Taylor returned to government as Senior Advisor to the Commissioner of the US Food and Drug Administration for the Obama administration.
The Canadian Biotechnology Advisory Committee that reviewed Canada's regulations in 2003 was accused by environmental and citizen groups of not representing the full spectrum of public interests and for being too closely aligned to industry groups.
Most of the Chinese National Biosafety Committee are involved in biotechnology leading to criticisms that they do not represent a wide enough range of public concerns.
Religion
Main article: Religious views on genetically modified foodsNo GM foods have yet been designated as unacceptable by religious authorities.
Other
Industrial agriculture
GM crops play a key role in contemporary large scale agriculture, which involves monoculture, heavy use of herbicides and pesticides, use of equipment that requires large amounts of fossil fuels, and heavy water use. The market for organic food products has grown substantially worldwide and in the US, driven to a great extent by concern over the healthfulness of the products of industrial agriculture and by environmental concerns, as described in the article on organic food. Vandana Shiva, the founder of the group Navdanya, is an example of those who protest this paradigm: "We need biodiversity intensification that works with nature's nutrient and water cycles, not against them."
Africa
In 2002, Zambia refused emergency food aid from developed countries, fearing that the food is unsafe. During a conference in the Ethiopian capital of Addis Ababa, Kingsley Amoako, Executive Secretary of the United Nations Economic Commission for Africa (UNECA), encouraged African nations to accept genetically modified food and expressed dissatisfaction in the public's negative opinion of biotechnology.
Litigation in the US
Four federal district court suits have been brought against Animal and Plant Health Inspection Service (APHIS), the agency within USDA that regulates genetically modified plants. Two involved field trials (herbicide-tolerant turfgrass in Oregon; pharmaceutical-producing corn and sugar in Hawaii) and two the deregulation of GM alfalfa. and GM sugar beet. Initially APHIS lost all four cases, with the judges ruling they failed to diligently follow the guidelines set out in the National Environmental Policy Act. However, the Supreme Court overturned the nationwide ban on GM alfalfa and an appeal court allowed the partial deregulation of GM sugar beet crops. After APHIS prepared Environmental Impact Statements for both alfalfa and sugar beet they were deregulated again.
India
Controversies over GM crops and GM food in India have recapitulated many of the issues discussed in this article, but have unique aspects as well. In India, GM cotton yields in Maharashtra, Karnataka, and Tamil Nadu had an average 42% increase in yield with GM cotton in 2002, the first year of commercial GM cotton planting. However, there was a severe drought in Andhra Pradesh that year and the parental cotton plant used in the genetic engineered variant was not well suited to extreme drought, so Andhra Pradesh saw no increase in yield. Drought resistant variants were developed and, with the substantially reduced losses to insect predation, by 2011 88% of Indian cotton was GM. Though disputed the economic and environmental benefits of GM cotton in India to the individual farmer have been documented. A long-term study (2002 through 2008) on the economic impacts of Bt cotton in India, published in the Journal PNAS in 2012, showed that Bt cotton increased yields, profits, and living standards of smallholder farmers. However, recently cotton bollworm has been developing resistance to Bt cotton and the Indian Agriculture Ministry linked farmers' suicides in India to the declining performance of Bt cotton for the first time. Consequently, in 2012 the state of Maharashtra banned Bt cotton and ordered a socio-economic study of its use by independent institutes. Indian regulators cleared the Bt brinjal, a genetically modified eggplant, for commercialisation in October 2009. Following opposition from some scientists, farmers and environmental groups, a moratorium was imposed on its release in February 2010 "for as long as it is needed to establish public trust and confidence".
On 1 January 2013, a new law came into effect that required all packaged foods containing any genetically modified organisms to be labeled as such. The Legal Metrology (Packaged Commodities) Rules, 2011 states that "every package containing the genetically modified food shall bear at the top of its principal display panel the letters GM." The rules apply to 19 products including biscuits, breads, cereals and pulses, and a few others. The law faced criticism from consumer rights activists as well as from the packaged food industry; both sides had major concerns that no logistical framework or regulations had been established to guide implementation and enforcement of the law.
Availability of GM seed for testing
The value of current independent studies is considered by some to be problematic because, due to restrictive end-user agreements, independent researchers cannot obtain GM plants to study. Cornell University's Elson Shields, the spokesperson for a group of scientists who oppose this practice, submitted a statement to the United States Environmental Protection Agency (EPA) protesting that "as a result of restrictive access, no truly independent research can be legally conducted on many critical questions regarding the technology". Scientific American noted that several studies that were initially approved by seed companies were later blocked from publication when they returned "unflattering" results. While recognising that seed companies' intellectual property rights need to be protected, Scientific American calls the practice dangerous and has called for the restrictions on research in the end-user agreements to be lifted immediately and for the EPA to require, as a condition of approval, that independent researchers have unfettered access to GM products for testing. In February 2009, the American Seed Trade Association (ASTA) agreed that they "would allow researchers greater freedom to study the effects of GM food crops." This agreement left many scientists optimistic about the future, but there is little optimism as to whether this agreement has the ability to "alter what has been a research environment rife with obstruction and suspicion."
Biological process
The use of genetically modified organisms has sparked significant controversy in many areas. Some groups or individuals see the generation and use of GMO as intolerable meddling with biological states or processes that have naturally evolved over long periods of time, while others are concerned about the limitations of modern science to fully comprehend all of the potential negative ramifications of genetic manipulation. Other people see genetic engineering as a continuation in the role humanity has occupied for thousands of years in selective breeding.
GMOs' proponents note that because of the safety testing requirements imposed on GM foods, the risk of introducing a plant variety with a new allergen or toxin using genetic modification is much smaller than using traditional breeding processes. Transgenic genetic engineering can have less impact on the expression of genomes or on protein and metabolite levels than conventional breeding or plant (non-directed) mutagenesis. Toxicologists note that "conventional food is not risk-free; allergies occur with many known and even new conventional foods. For example, the kiwi fruit was introduced into the U.S. and the European markets in the 1960s with no known human allergies; however, today there are people allergic to this fruit."
Protests
In 1983, a biotech company, Advanced Genetic Sciences (AGS) applied for U.S. government authorization to perform field tests with the ice-minus strain of P. syringae, but environmental groups and protestors delayed the field tests for four years with legal challenges. In 1987, the ice-minus strain of P. syringae became the first genetically modified organism (GMO) to be released into the environment when a strawberry field in California was sprayed with the ice-minus strain of P. syringae. The results were promising, showing lowered frost damage to the treated plants. Dr. Lindow also conducted an experiment on a crop of potato seedlings sprayed with ice-minus P. syringae. He was successful in protecting the potato crop from frost damage with a strain of ice-minus P. syringae. Both test fields were attacked by activist groups the night before the tests occurred: "The world's first trial site attracted the world's first field trasher."
Concern about gene flow drives some protesters. In May 2012, a group called "Take the Flour Back" led by Gerald Miles protested against plans by a group from Rothamsted Experimental Station, based in Harpenden, Hertfordshire, England, to stage an experimental trial to use genetically modified wheat to repel aphids. The researchers, led by John Pickett, wrote a letter to the group "Take the Flour Back" in early May 2012, asking them to call off their protest, aimed for 27 May 2012. One of the members of Take the Flour Back, Lucy Harrap, said that the group was concerned about spread of the crops into nature, and cited examples of outcomes in the United States and Canada. Rothamsted Research and Sense About Science ran question and answer sessions with scientists about issues of contamination.
Within the UK and many other European countries many trial crops have been destroyed by protesters: for public research experiments alone, 80 acts of destruction have been compiled. The protesters claim the destruction of the crops creates opportunities to be heard. The primary concern of the campaigners though is contamination of existing crops could destroy existing markets. Scientists take many precautions to minimise the risks as much as possible and admit the risk of contamination is small. However, campaigners counter with examples of widespread contamination that has already occurred despite assurances and promises from scientists. The scientists give several reasons for the need for trials - climate change, a growing global population and reduced use of chemicals. The campaigners draw attention to natural and organic solutions to reduce chemical use and question the usefulness of the trials (e.g. field trials in the UK for a crop designed for Africa).
See also
- Arpad Pusztai
- Corngate
- Genetic pollution
- Environmental issues with agriculture
- Ice-minus bacteria
- Nayakrishi
- Seralini affair
References
- AAAS Board of Directors (2012) Legally Mandating GM Food Labels Could Mislead and Falsely Alarm Consumers
- World Health Organization 20 questions on genetically modified foods Accessed December 22, 2012
- ^ Dr. Christopher Preston AgBioWorld 2011. Peer Reviewed Publications on the Safety of GM Foods
- ^ NRC. (2004). Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. National Academies Press. Free full-text. See pp11ff on need for better standards and tools to evaluate GM food.
- ^ Winter CK and Gallegos LK. (2006) Safety of Genetically Engineered Food. University of California Agriculture and Natural Resources Communications Publication 8180.
- Pamela Ronald (2011) Genetically Engineered Crops—What, How and Why
- Associated Press. Alicia Chang (2012) California voters rebuff labels on GMO foods
- ^ Key S, Ma JK, Drake PM (2008). "Genetically modified plants and human health". J R Soc Med. 101 (6): 290–8. doi:10.1258/jrsm.2008.070372. PMC 2408621. PMID 18515776.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ REPORT 2 of the Council on Science and Public Health. (2012) Labeling of Bioengineered Foods
- ^ Hunt, Lesley (2004). "Factors determining the public understanding of GM technologies" (Review Article). AgBiotechNet. 6 (128): 1–8.
- Lazarus, Richard J (1991). "The Tragedy of Distrust in the Implementation of Federal Environmental Law". Law and Contemporary Problems. 54 (4): 311–74. doi:10.2307/1191880. JSTOR 1191880.
- Memo from The Mellman Group, Inc. to The Pew Initiative On Food And Biotechnology, 16 November 2006. Review Of Public Opinion Research
- Jennie Addario. Ryerson Review of Journalism. Spring, 2002. Horror Show: Why the debate over genetically modified organisms and other complex science stories freak out newspapers
- Example of protester confusion. Sara Chamberlain. New Internationalist Magazine. Issued 293. Published on 5 August 1997 "Sara Chamberlain Dissects The Food That We Eat And Finds Some Alarming Ingredients. Article On Genetically Engineered/modified Foods For New Internationalist Magazine" Quote: "What would you think if I said that your dinner resembles Frankenstein an unnatural hodgepodge of alien ingredients? Fish genes are swimming in your tomato sauce, microscopic bacterial genes in your tortillas, and your veg curry has been spiked with viruses."
- Deloitte 2010 Food Survey Genetically Modified Foods retrieved 10 October 2012
- "Opposition decreasing or acceptance increasing?: An overview of European consumer polls on attitudes to GMOs". GMO Compass. 16 April 2009. Retrieved 10 October 2012.
- Gaskell G et al October 2010. Europeans and Biotechnology in 2010: Winds of change? A report to the European Commission’s Directorate-General for Research European Commission Directorate-General for Research 2010 Science in Society and Food, Agriculture & Fisheries, & Biotechnology, EUR 24537 EN
- Gaskell G, Allansdottir A, Allum N, Castro P, Esmer Y, Fischler C, Jackson J, Kronberger N, Hampel J, Mejlgaard N, Quintanilha A, Rammer A, Revuelta G, Stares S, Torgersen H, Wager W (2011). "The 2010 Eurobarometer on the life sciences". Nat. Biotechnol. 29 (2): 113–4. doi:10.1038/nbt.1771. PMID 21301431.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Genetically modified (GM) foods". Food Standards Australia and New Zealand. 4 October 2012. Retrieved 5 November 2012.
- "Consumer Attitudes Survey 2007, A benchmark survey of consumers' attitudes to food issues". Food Standards Australia New Zealand. 2008. Retrieved 5 November 2012.
{{cite web}}: Unknown parameter|month=ignored (help). - "Say no to genetic engineering". Greenpeace.
- "Genetic engineerinf". Friends of the Earth.
- "GE-Agriculture". The Institute of Science in Society.
- "About GMWatch". GMWatch.
- Keith Kloor for Slate Magazine. 26 September 2012 GMO Opponents Are the Climate Skeptics of the Left
- Knight, Peter (2003). Conspiracy Theories in American History. ABC-CLIO. p. 310. ISBN 1576078124.
{{cite book}}: Invalid|ref=harv(help) - van Eijck, Paul (10 March 2010). "The History and Future of GM Potatoes". PotatoPro.
- ^ Winter, CK and Gallegos, LK. 2006. University of California Agricultural and Natural Resource Service. ANR Publication 8180. Safety of Genetically Engineered Food
- EFSA Panel on Genetically Modified Organisms (GMO) (2011) EFSA Journal 9(5) 2150 . Guidance for risk assessment of food and feed from genetically modified plants
- ^ The Editors. A Seedy Practice Scientific American 301(2) 22, August 2009
- Organisation for Economic Co-operation and Development. Report of the Task Force for the Safety of Novel Foods and Feeds C(2000)86/ADD1. 17 May 2000 page 4, paragraph 4.
- AAAS Board of Directors. (2012) Legally Mandating GM Food Labels Could Mislead and Falsely Alarm Consumers.
- A decade of EU-funded GMO research (2001-2010) (pdf). Directorate-General for Research and Innovation. Biotechnologies, Agriculture, Food. European Union. 2010. p. 16. doi:10.2777/97784. ISBN 978-92-79-16344-9.
- CORDIS - Community Research and Development Information Service. 2005-01-06 EU project publishes conclusions and recommendations on GM foods
- König A, Cockburn A, Crevel RW, Debruyne E, Grafstroem R, Hammerling U, Kimber I, Knudsen I, Kuiper HA, Peijnenburg AA, Penninks AH, Poulsen M, Schauzu M, Wal JM (2004). "Assessment of the safety of foods derived from genetically modified (GM) crops". Food Chem. Toxicol. 42 (7): 1047–88. doi:10.1016/j.fct.2004.02.019. PMID 15123382.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ OECD (2010) Consensus Document on Molecular Characterisation of Plants Derived from Modern Biotechnology
- EFSA Panel on Genetically Modified Organisms (GMO) (2012). "Scientific opinion addressing the safety assessment of plants developed through cisgenesis and intragenesis". EFSA Journal. 10 (2): 12561. doi:10.2903/j.efsa.2012.2561.
- Diels, Johan (2011). "Association of financial or professional conflict of interest to research outcomes on health risks or nutritional assessment studies of genetically modified products". Food Policy. 36: 197–203. doi:10.1016/j.foodpol.2010.11.016.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - "Safety Evaluation of Foods Derived by Modern Biotechnology: Concepts and Principles" (PDF). Organisation for Economic Co-operation and Development. Retrieved 21 June 2009.
- UK GM expert calls for tougher tests BBC 7 September 1999
- Millstone E, Brunner E, Mayer S (1999). "Beyond 'substantial equivalence'". Nature. 401 (6753): 525–6. doi:10.1038/44006. PMID 10524614.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Burke D (1999). "No GM conspiracy". Nature. 401 (6754): 640–1. doi:10.1038/44262. PMID 10537098.
{{cite journal}}: Unknown parameter|month=ignored (help) - Trewavas A, Leaver CJ (1999). "Conventional crops are the test of GM prejudice". Nature. 401 (6754): 640. doi:10.1038/44258. PMID 10537097.
{{cite journal}}: Unknown parameter|month=ignored (help) - Gasson MJ (1999). "Genetically modified foods face rigorous safety evaluation". Nature. 402 (6759): 229. doi:10.1038/46147. PMID 10580485.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kuiper HA, Kleter GA, Noteborn HP, Kok EJ (2002). "Substantial equivalence--an appropriate paradigm for the safety assessment of genetically modified foods?". Toxicology. 181–182: 427–31. doi:10.1016/S0300-483X(02)00488-2. PMID 12505347.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Barbara Keeler and Marc Lappe "Some Food for FDA Regulation" Los Angeles Times,7 January 2001
- Jennifer Ackerman for National Geographic magazine. May 2002 Genetically Modified Foods
- OECD harmonization webpage
- Ricroch, AE, Bergé, JB, Kuntz, M. Evaluation of genetically engineered crops using transcriptomic, proteomic and metabolomic profiling techniques. Plant Physiology (2011) vol. 155(4) 1752-1761. http://dx.doi.org/10.1104/pp.111.173609
- Herman, RA, Price, WD. Unintended Compositional Changes in Genetically Modified (GM) Crops: 20 Years of Research. Journal of Agricultural and Food Chemistry (2013). http://pubs.acs.org/doi/abs/10.1021/jf400135r.
- Staff, GMO Compass. February 15, 2006. Food Safety Evaluation: The Allergy Check
- Drake Bennett Our allergies, ourselves Boston Globe 7 May 2006
- Lehrer SB, Bannon GA (2005). "Risks of allergic reactions to biotech proteins in foods: perception and reality". Allergy. 60 (5): 559–64. doi:10.1111/j.1398-9995.2005.00704.x. PMID 15813800.
{{cite journal}}: Unknown parameter|month=ignored (help) - Nordlee JA, Taylor SL, Townsend JA, Thomas LA, Bush RK (1996). "Identification of a Brazil-nut allergen in transgenic soybeans". N. Engl. J. Med. 334 (11): 688–92. doi:10.1056/NEJM199603143341103. PMID 8594427.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Warren Leary Genetic Engineering of Crops Can Spread Allergies, Study Shows New York Times Thursday, 14 March 1996
- Streit, L.G.; et al. (2001). "Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits". Crop Sci. 41 (6): 1757–1760. doi:10.2135/cropsci2001.1757.
- Prescott VE, Campbell PM, Moore A, Mattes J, Rothenberg ME, Foster PS, Higgins TJ, Hogan SP (2005). "Transgenic expression of bean alpha-amylase inhibitor in peas results in altered structure and immunogenicity". J. Agric. Food Chem. 53 (23): 9023–30. doi:10.1021/jf050594v. PMID 16277398.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Herman EM (2003). "Genetically modified soybeans and food allergies". J. Exp. Bot. 54 (386): 1317–9. doi:10.1093/jxb/erg164. PMID 12709477.
{{cite journal}}: Unknown parameter|month=ignored (help) - Herman EM, Helm RM, Jung R, Kinney AJ (2003). "Genetic modification removes an immunodominant allergen from soybean". Plant Physiol. 132 (1): 36–43. doi:10.1104/pp.103.021865. PMC 1540313. PMID 12746509.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bhalla PL, Swoboda I, Singh MB (1999). "Antisense-mediated silencing of a gene encoding a major ryegrass pollen allergen". Proc. Natl. Acad. Sci. U.S.A. 96 (20): 11676–80. doi:10.1073/pnas.96.20.11676. PMC 18093. PMID 10500236.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1051/ebr:2008014, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1051/ebr:2008014instead. - ^ Flachowsky G, Chesson A, Aulrich K (2005). "Animal nutrition with feeds from genetically modified plants". Arch Anim Nutr. 59 (1): 1–40. doi:10.1080/17450390512331342368. PMID 15889650.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Beagle JM, Apgar GA, Jones KL, Griswold KE, Radcliffe JS, Qiu X, Lightfoot DA, Iqbal MJ (2006). "The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs". J. Anim. Sci. 84 (3): 597–607. PMID 16478951.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Brigulla M, Wackernagel W (2010). "Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues". Appl. Microbiol. Biotechnol. 86 (4): 1027–41. doi:10.1007/s00253-010-2489-3. PMID 20191269.
{{cite journal}}: Unknown parameter|month=ignored (help) - Guertler P, Paul V, Albrecht C, Meyer HH (2009). "Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk". Anal Bioanal Chem. 393 (6–7): 1629–38. doi:10.1007/s00216-009-2667-2. PMID 19225766.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/cr.2011.158, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/cr.2011.158instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0734-9750(00)00033-1, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0734-9750(00)00033-1instead. - Netherwood T, Martín-Orúe SM, O'Donnell AG, Gockling S, Graham J, Mathers JC, Gilbert HJ (2004). "Assessing the survival of transgenic plant DNA in the human gastrointestinal tract". Nat. Biotechnol. 22 (2): 204–9. doi:10.1038/nbt934. PMID 14730317.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S1360-1385(98)01251-5, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S1360-1385(98)01251-5instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1080/10937400306469, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1080/10937400306469instead. - Snell C, Bernheim A, Bergé JB, Kuntz M, Pascal G, Paris A, Ricroch AE (2012). "Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review" (PDF). Food Chem. Toxicol. 50 (3–4): 1134–48. doi:10.1016/j.fct.2011.11.048. PMID 22155268.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Magaña-Gómez JA, de la Barca AM (2009). "Risk assessment of genetically modified crops for nutrition and health". Nutr. Rev. 67 (1): 1–16. doi:10.1111/j.1753-4887.2008.00130.x. PMID 19146501.
{{cite journal}}: Unknown parameter|month=ignored (help) - Dona A, Arvanitoyannis IS (2009). "Health risks of genetically modified foods". Crit Rev Food Sci Nutr. 49 (2): 164–75. doi:10.1080/10408390701855993. PMID 18989835.
{{cite journal}}: Unknown parameter|month=ignored (help) - Dona, Artemis et al (2009) Health Risks of Genetically Modified Foods Full version of paper in Critical Reviews in Food Science and Nutrition, 49:164–175 (2009). Retrieved 28 October 2010
- Amman Klaus (2009) Human and Animal Health - Rebuttal to a Review of Dona and Arvanitoyannis 2009, part one European Federation of Biotechnology, 31 August 2009. Retrieved 28 October 2010
- Amman, Klaus (2009) Rebuttal to a review of Dona and Arvanitoyannis 2009 Retrieved on 28 October 2010
- Craig, Richard (2010) Response to "Health Risks of Genetically Modified Foods" from Dona and Arvanitoyannis (2009) in Critical Reviews in Food Science and Nutrition (49:164-175) Critical reviews in food science and nutrition, 2010, vol. 50, no 1, pp. 85–91. Retrieved 28 October 2010
- Aumaitre A (2004). "Safety assessment and feeding value for pigs, poultry and ruminant animals of pest protected (Bt) plants and herbicide tolerant (glyphosate, glufosinate) plants: interpretation of experimental results observed worldwide on GM plants". Italian Journal of Animal Science. 3 (2): 107–121.
- Domingo JL (2007). "Toxicity studies of genetically modified plants: a review of the published literature". Crit Rev Food Sci Nutr. 47 (8): 721–33. doi:10.1080/10408390601177670. PMID 17987446.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nbt0607-624b, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nbt0607-624binstead. - Vain, Philippe (2007) Trends in GM crop, food and feed safety literature (2007)
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.envint.2011.01.003, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.envint.2011.01.003instead. - Physicians and Scientists for Responsible Application of Science and Technology Official Website
- Staff, United States General Accounting Office. May 23, 2002. GAO-02-566 Report to Congressional Requesters: Genetically Modified Foods pp 30-32
- FAO/WHO (2000b) Safety Aspects of Genetically Modified Foods of Plant Origin. Report of a Joint FAO/WHO Expert Consultation on Foods Derived from Biotechnology (Geneva, Switzerland, May 29 –June 2, 2000).
- Wendler, David. First published Fri Jan 30, 2009; substantive revision Thu Sep 20, 2012 The Ethics of Clinical Research The Stanford Encyclopedia of Philosophy (Fall 2012 Edition), Edward N. Zalta (ed.)
- Germolec D.R.; et al. (2003). "Key issues for the assessment of the allergenic potential of genetically modified foods: breakout group reports". Environ Health Perspect. 111 (8): 1131–1139. PMC 1241563. PMID 12826486.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Tang G, et al (2009) Golden Rice is an effective source of vitamin A. Am J Clin Nutr. 89(6) 1776-83.
- Tang G; et al. (2012). "β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children". Am J Clin Nutr. 96 (3): 658–64. doi:10.3945/ajcn.111.030775. PMID 22854406.
{{cite journal}}: Explicit use of et al. in:|author=(help) - By Corinne Segal for the Tufts Daily. September 17, 2012, Updated September 20, 2012 Alleged ethics violations surface in Tufts-backed study
- ^ Ewen SW, Pusztai A (1999). "Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine". Lancet. 354 (9187): 1353–4. doi:10.1016/S0140-6736(98)05860-7. PMID 10533866.
{{cite journal}}: Unknown parameter|month=ignored (help) - Vasconcelos IM, Oliveira JT. Antinutritional properties of plant lectins. Toxicon. 2004 Sep 15;44(4) 385-403.
- Staff, Rowett Research Institute Press Office. Rowett Research Institute: Audit Report Overview
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.281.5380.1124b, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.281.5380.1124binstead. - Randerson J. (2008). Arpad Pusztai: Biological divide. The Guardian.
- Bourne, F.J., et al (1998) Audit Report Overview Rowett Research Institute, 28 October 1998. Retrieved 28 November 2010
- Murray, Noreen et al, (1999) Review of data on possible toxicity of GM potatoes The Royal Society, 1 June 1999. Retrieved 28 November 2010
- ^ Enserink M (1999). "Transgenic food debate. The Lancet scolded over Pusztai paper". Science. 286 (5440): 656. doi:10.1126/science.286.5440.656a. PMID 10577214.
{{cite journal}}: Unknown parameter|month=ignored (help) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0140-6736(99)00341-4, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0140-6736(99)00341-4instead. - J.M. Rhodes (199). "Genetically modified foods and the Pusztai affair". BMJ. 8 (318): 1284.
- Séralini GE, Cellier D, de Vendomois JS (2007). "New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity". Arch. Environ. Contam. Toxicol. 52 (4): 596–602. doi:10.1007/s00244-006-0149-5. PMID 17356802.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - De Vendômois JS, Roullier F, Cellier D, Séralini GE (2009). "A comparison of the effects of three GM corn varieties on mammalian health". Int J Biol Sci. 5 (7): 706–26. PMC 2793308. PMID 20011136.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Seralini GE; et al. (2011). "Genetically modified crops safety assessments: present limits and possible improvements". Environmental Sciences Europe. 23: 10.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Statement of the Scientific Panel on Genetically Modified Organisms on the analysis of data from a 90-day rat feeding study with MON 863 maize
- "EFSA review of statistical analyses conducted for the assessment of the MON 863 90-day rat feeding study". EFSA Journal. 5 (6). doi:10.2903/j.efsa.2007.19r.
- "EFSA Minutes of the 55th Plenary Meeting of the Scientific Panel on Genetically Modified Organisms Held on 27–28 January 2010 IN Parma, Italy, Annex 1, Vendemois et al 2009" (PDF). European Food Safety Authority report. Retrieved 11 November 2010.
- EFSA Scientific Committee (2011)EFSA guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed. EFSA Journal 2011;9(12) 2438
- "Review of the report by Séralini et al., (2007): "New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity"". FSANZ final assessment report. Retrieved 11 November 2010.
- "FSANZ reaffirms its risk assessment of genetically modified corn MON 863". FSANZ fact sheets 2007. 25 July 2010. Retrieved 11 November 2010.
- "Feeding studies and GM corn MON863". Food Standards Australia New Zealand. 2012. Retrieved 10 October 2012.
{{cite web}}: Unknown parameter|month=ignored (help) - Doull J, Gaylor D, Greim HA, Lovell DP, Lynch B, Munro IC (2007). "Report of an Expert Panel on the reanalysis by of a 90-day study conducted by Monsanto in support of the safety of a genetically modified corn variety (MON 863)". Food Chem. Toxicol. 45 (11): 2073–85. doi:10.1016/j.fct.2007.08.033. PMID 17900781.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Opinion relating to the deposition of 15 December 2009 by the Member of Parliament, François Grosdidier, as to the conclusions of the study entitled "A comparison of the effects of three GM corn varieties on mammalian health"". English translation of French High Council of Biotechnologies Scientific Committee document. Retrieved 11 November 2010.
- ^ Séralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS (2012). "Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize". Food Chem. Toxicol. 50 (11): 4221–31. doi:10.1016/j.fct.2012.08.005. PMID 22999595.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Thomas Lumley for Stats Chat website. 20 September 2012 Roundup scare
- "Poison postures". Nature. 489 (7417): 474. 2012. doi:10.1038/489474a. PMID 23025010.
{{cite journal}}: Unknown parameter|month=ignored (help) - Séralini, Gilles-Eric (2012). Tous Cobayes !: OGM, pesticides et produits chimiques. Editions Flammarion. ISBN 9782081262362.
- "Tous cobayes? (2012) - IMDb". IMDB. IMDB.com.
- Carl Zimmer on Discovery Magazine blog, The Loom. 21 September 2012 From Darwinius to GMOs: Journalists Should Not Let Themselves Be Played
- Andrew Kniss for Control Freaks Blog. 19 September 2012 Explanation of rat study
- Suzuki H, Mohr U, Kimmerle G (1979). "Spontaneous endocrine tumors in Sprague-Dawley rats". J. Cancer Res. Clin. Oncol. 95 (2): 187–96. PMID 521452.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Mortality and In-Life Patterns in Sprague-Dawley" (PDF). Huntingdon Life Sciences. Retrieved 26 October 2012.
- ^ "Sprague Dawley" (PDF). Harlan. Retrieved 26 October 2012.
- Declan Butler for Nature News, 10 October 2012. Hyped GM maize study faces growing scrutiny: Food-safety bodies slam feeding study that claims increased cancer incidence in rats.
- "UPDATE 3-Study on Monsanto GM corn concerns draws scepticism". Reuters. 19 September 2012.
- By Ben Hirschler and Kate Kelland. Reuters "Study on Monsanto GM corn concerns draws skepticism" 20 September 2012
- MacKenzie, Deborah (19 September 2012) Study linking GM crops and cancer questioned New Scientist. Retrieved 26 September 2012
- Elizabeth Finkel (9 October 2012). "GM corn and cancer: the Séralini affai".
- Tim Carman for the Washington Post. Posted at 07:30 PM ET, 19 September 2012. French scientists question safety of GM corn
- Avis des Académies nationales d’Agriculture, de Médecine, de Pharmacie, des Sciences, des Technologies, et Vétérinaire sur la publication récente de G.E. Séralini et al. sur la toxicité d’un OGM Communiqué de presse 19 octobre 2012
- Food and Chemical Toxicology | Articles in Press | ScienceDirect.com
- Staff (1 October 2012) A study of the University of Caen neither constitutes a reason for a re-evaluation of genetically modified NK603 maize nor does it affect the renewal of the glyphosate approval German Federal Institute for Risk Assessment (BfR). Retrieved 14 October 2012
- Staff (5 October 2012) BVL prüft Rattenfütterungsstudie mit gentechnisch verändertem Mais und glyphosathaltigen Pflanzenschutzmitteln (Seralini et al. 2012) (in German) "BVL checks rat feeding study with a genetically modified maize and glyphosate pesticide (Seralini et al 2012.)", The German Federal Office of Consumer Protection and Food Safety (BVL). Retrieved 14 October 2012
- Staff (22 October 2012) French panel rejects study linking GM corn to cancer Agence France Presse. Retrieved 23 October 2012
- Staff (8 October 2012) VIB concludes that Séralini study is not substantiated VIB Life Sciences Research Institute, Belgium. Retrieved 14 October 2012
- Staff (October 2012) The Technical University of Denmark National Food Institute's assessment of a new long-term trial with genetically modified maize NK603 and spray Roundup (In Danish) Technical University of Denmark, Danish National Food Institute, Rertrieved 23 October 2012
- Staff (October 2012) Response to Séralini paper on the long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize Food Standards Australia New Zealand. Retrieved 14 October 2012
- Garcia, Jose Fernando et al (2012) CTNBio Considered Opinion on Sep. 2012 publication of Seralini et al Brazilian Ministry of Science Technology and Innovation, National Biosafety Technical Commission, Retrieved 7 December 2012
- EFSA, 4 October 2012. Press release with summary of findings. Full review: EFSA (2012) Review of the Séralini et al. (2012) publication on a 2-year rodent feeding study with glyphosate formulations and GM maize NK603 as published online on 19 September 2012 in Food and Chemical Toxicology EFSA Journal 2012;10(10) 2910 doi:10.2903/j.efsa.2012.2910
- Séralini GE, Mesnage R, Defarge N (2013). "Answers to critics: Why there is a long term toxicity due to a Roundup-tolerant genetically modified maize and to a Roundup herbicide". Food Chem. Toxicol. 53: 476–483. doi:10.1016/j.fct.2012.11.007. PMID 23146697.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Aris A, Leblanc S (2011). "Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada". Reprod. Toxicol. 31 (4): 528–33. doi:10.1016/j.reprotox.2011.02.004. PMID 21338670.
{{cite journal}}: Unknown parameter|month=ignored (help) - Poulter, Sean (20 May 2011). "GM food toxins found in the blood of 93% of unborn babies". Daily Mail. Retrieved 7 February 2012.
- "Many Women, no Cry - OGM : environnement, santé et politique" (in English and French). Marcel-kuntz-ogm.over-blog.fr. 16 January 2012. Retrieved 7 February 2012.
{{cite web}}: CS1 maint: unrecognized language (link) - "FSANZ response to study linking Cry1Ab protein in blood to GM foods". Food Standards Australia New Zealand. 27 May 2011. Retrieved 10 October 2012.
- "FSANZ response to study linking Cry1Ab protein in blood to GM foods". FSANZ.
- ^ Conner AJ, Glare TR, Nap JP (2003). "The release of genetically modified crops into the environment. Part II. Overview of ecological risk assessment". Plant J. 33 (1): 19–46. doi:10.1046/j.0960-7412.2002.001607.x. PMID 12943539.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Wolfenbarger LL, Phifer PR (2000). "The ecological risks and benefits of genetically engineered plants". Science. 290 (5499): 2088–93. doi:10.1126/science.290.5499.2088. PMID 11118136.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brookes, Graham and Barfoot, Peter (May 2012) GM crops: global socio-economic and environmental impacts 1996-2010 PG Economics Ltd. UK, Retrieved 3 January 2012
- "History of Bt". University of California. Retrieved 8 February 2010.
- Hall, H. "Bt corn: is it worth the risk?". The Science Creative Quarterly.
- Dorsch, J.A. et al. Cry1a Toxins of Bacillus Thuringiensis Bind Specifically to a Region Adjacent to the Membrane-Proximal Extracellular Domain of Bt-R-1 in Manduca Sexta: Involvement of a Cadherin in the Entomopathogenicity of Bacillus Thuringiensis. Insect Biochemistry and Molecular Biology 32, 1025-1036 (2002)
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/s11248-010-9446-x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/s11248-010-9446-xinstead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nbt1381, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nbt1381instead. - Losey JE et al. (1999) Transgenic pollen harms monarch larvae. Nature 399: 214
- Sears MK, Hellmich RL, Stanley-Horn DE, Oberhauser KS, Pleasants JM, Mattila HR, Siegfried BD, Dively GP (2001). "Impact of Bt corn pollen on monarch butterfly populations: a risk assessment". Proc. Natl. Acad. Sci. U.S.A. 98 (21): 11937–42. Bibcode:2001PNAS...9811937S. doi:10.1073/pnas.211329998. JSTOR 3056827. PMC 59819. PMID 11559842.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gatehouse AM, Ferry N, Raemaekers RJ (2002). "The case of the monarch butterfly: a verdict is returned". Trends Genet. 18 (5): 249–51. doi:10.1016/S0168-9525(02)02664-1. PMID 12047949.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Lövei GL, Andow DA, Arpaia S (2009). "Transgenic insecticidal crops and natural enemies: a detailed review of laboratory studies". Environ. Entomol. 38 (2): 293–306. doi:10.1603/022.038.0201. PMID 19389277.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Shelton AM, Naranjo SE, Romeis J, Hellmich RL, Wolt JD, Federici BA, Albajes R, Bigler F, Burgess EP, Dively GP, Gatehouse AM, Malone LA, Roush R, Sears M, Sehnal F (2009). "Setting the record straight: a rebuttal to an erroneous analysis on transgenic insecticidal crops and natural enemies". Transgenic Res. 18 (3): 317–22. doi:10.1007/s11248-009-9260-5. PMID 19357987.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Carpenter JE (2011). "Impact of GM crops on biodiversity". GM Crops. 2 (1): 7–23.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.soilbio.2007.11.002,, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.soilbio.2007.11.002,instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1098/rspb.2004.3049, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1098/rspb.2004.3049instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.agee.2004.03.005, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.agee.2004.03.005instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1098/rspb.2006.3522, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1098/rspb.2006.3522instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.agee.2006.05.012, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.agee.2006.05.012instead. - Pleasants JM and Oberhauser KS (2012) Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population Insect Conservation and Diversity (2012) doi: 10.1111/j.1752-4598.2012.00196.x (Article first published online: 12 March 2012)
- Andre Pollack for the New York Times, 11 July 2011. In Midwest, Flutters May Be Far Fewer
- Relyea RA (2005). "The Impact of Insecticides and Herbicides on The Biodiversity and Productivity of Aquatic Communities" (PDF). Ecological Applications. 15 (2): 618–627.
- Robin Meadows (2005)Common Herbicide Lethal to Wetland Species Conservation Magazine 6(3)
- Lu Y, Wu K, Jiang Y, Xia B, Li P, Feng H, Wyckhuys KA, Guo Y (2010). "Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China". Science. 328 (5982): 1151–4. doi:10.1126/science.1187881. PMID 20466880.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Lang, Susan (25 July 2006). "Profits die for Bt cotton in China". Cornell Chronicle. Retrieved 10 October 2012.
- Wang, Shenghui; Just, David R.; Per, Pinstrup-Andersen (2008). "Bt-cotton and secondary pests". International Journal of Biotechnology. 10 (2/3): 113–21. doi:10.1504/IJBT.2008.018348.
- Wang, Zi-jun; Lin, Hai; Huang, Ji-kun; Hu, Rui-fa; Rozelle, Scott; Pray, Carl (2009). "Bt Cotton in China: Are Secondary Insect Infestations Offsetting the Benefits in Farmer Fields?". Agricultural Sciences in China. 8: 83–90. doi:10.1016/S1671-2927(09)60012-2.
- Zhao JH, Ho P, Azadi H (2012). "Erratum to: Benefits of Bt cotton counterbalanced by secondary pests? Perceptions of ecological change in China". Environ Monit Assess. doi:10.1007/s10661-012-2699-5. PMID 22864609.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Goswami, Bhaskar (2007). "Making a meal of Bt cotton". InfoChange. Retrieved 10 October 2012.
{{cite web}}: Unknown parameter|month=ignored (help) - "Bug makes meal of Punjab cotton, whither Bt magic?". IANS. 2 September 2007. Retrieved 10 October 2012.
- Stone, Glenn Davis (2011). "Field versus Farm in Warangal: Bt Cotton, Higher Yields, and Larger Questions". World Development. 39 (3): 387–98. doi:10.1016/j.worlddev.2010.09.008.
- U.S. Department of Energy Genome Progrmas (2008). "Genetically Modified Foods and Organisms". Retrieved 15 November 2010.
- Andrew Pollack for the New York Times. "An Entrepreneur Bankrolls a Genetically Engineered Salmon" Published: 21 May 2012. Accessed 3 September 2012
- Eugene H. Buck, Specialist in Natural Resources Policy, Congressional Research ServiceGenetically Engineered Fish and Seafood: Environmental Concerns. 7 June 2011. Retrieved 3 September 2012.
- GMO Compass 12 December 2006 Genetically Modified Plants: Out-crossing and Gene Flow accessdate 23 April 2011
- GMO Compass. 5 June 2009 Mexico: controlled cultivation of genetically modified maize
- Mike Shanahan for Science and Development Network, 10 November 2004. Warning issued on GM maize imported to Mexico - SciDev.Net
- Katie Mantell for Science and Development Network, 30 November 2001 GM maize found ‘contaminating’ wild strains - SciDev.Net
- Quist D, Chapela IH (2001). "Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico". Nature. 414 (6863): 541–3. doi:10.1038/35107068. PMID 11734853.
{{cite journal}}: Unknown parameter|month=ignored (help) - Kaplinsky N, Braun D, Lisch D, Hay A, Hake S, Freeling M (2002). "Biodiversity (Communications arising): maize transgene results in Mexico are artefacts". Nature. 416 (6881): 601–2, discussion 600, 602. doi:10.1038/nature739. PMID 11935145.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Ortiz-Garcia, S. (2005). "Absence of detectable transgenes in local landraces of maize in Oaxaca, Mexico (2003-2004)". Proceedings of the National Academy of Sciences. 102 (35): 12338. doi:10.1073/pnas.0503356102.
- Piñeyro-Nelson A, Van Heerwaarden J, Perales HR, Serratos-Hernández JA, Rangel A, Hufford MB, Gepts P, Garay-Arroyo A, Rivera-Bustamante R, Alvarez-Buylla ER (2009). "Transgenes in Mexican maize: molecular evidence and methodological considerations for GMO detection in landrace populations". Mol. Ecol. 18 (4): 750–61. doi:10.1111/j.1365-294X.2008.03993.x. PMC 3001031. PMID 19143938.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Scientists play down 'superweed'" BBC, 25 July 2005 (source report)
- "First Wild Canola Plants With Modified Genes Found in United States". Arkansas Newswire. University of Arkansas. 6 August 2010. Retrieved 10 October 2012.
- Genetically Modified Canola 'Escapes' Farm Fields. NPR. Retrieved 8 February 2011.
- Black, Richard. (2010-08-06) BBC News – GM plants 'established in the wild'. Bbc.co.uk. Retrieved 8 February 2011.
- Eisberg, Neil GM crops are on the move Chemistry and Industry Ten Alps Publishing 7 November 2011 HighBeam Research accessed 7 July 2012
- William Neuman and Andrew Pollack (3 May 2010). "Farmers Cope With Roundup-Resistant Weeds". New York times.
- Report by the US National Academies "Genetically Engineered Crops Benefit Many Farmers, but the Technology Needs proper Management to Remain Effective" – press release on the report "The Impact of Genetically Engineered Crops on Farm Sustainability in the United States" Office of News and Public Information, News from the Academies, 13 April 2010. Retrieved 11 October 2010.
- Biotech Crops Are Good For Earth, Report Finds
- Marc Gunther for Fortune Magazine. 2 July 2007. Attack of the mutant rice
- APHIS Report of LibertyLink Rice Incidents
- Watrud LS, Lee EH, Fairbrother A, Burdick C, Reichman JR, Bollman M, Storm M, King G, Van de Water PK (2004). "Evidence for landscape-level, pollen-mediated gene flow from genetically modified creeping bentgrass with CP4 EPSPS as a marker". Proc. Natl. Acad. Sci. U.S.A. 101 (40): 14533–8. doi:10.1073/pnas.0405154101. PMC 521937. PMID 15448206.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Hamer, Ed; Anslow, Mark (1 March 2008). "10 reasons why organic can feed the world". Ecologist.
- ^ "Transgenic Crops: An Introduction and Resource Guide". Cls.casa.colostate.edu. Retrieved 8 March 2010.
- BBC News, Tuesday, 5 October 1999.. Terminator gene halt a 'major U-turn'
- "Nature World Conference on Science". Nature.com. 24 June 1999. Retrieved 8 March 2010.
- Haider Rizvi (Mar 21 2006). "BIODIVERSITY: Don't Sell "Suicide Seeds", Activists Warn". Inter Press Service.
{{cite news}}: Check date values in:|date=(help) - Shipitalo MJ, Malone RW, Owens LB (2008). "Impact of Glyphosate-Tolerant Soybean and Glufosinate-Tolerant Corn Production on Herbicide Losses in Surface Runoff". Journal of Environment Quality. 37 (2): 401–8. doi:10.2134/jeq2006.0540. PMID 18268303.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Shipitalo MJ, Malone RW, Owens LB (2008). "Impact of glyphosate-tolerant soybean and glufosinate-tolerant corn production on herbicide losses in surface runoff". J. Environ. Qual. 37 (2): 401–8. doi:10.2134/jeq2006.0540. PMID 18268303.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "How GMOs Unleashed a Pesticide Gusher". 3 October 2012.
- Roh JY, Choi JY, Li MS, Jin BR, Je YH (2007). "Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control". J. Microbiol. Biotechnol. 17 (4): 547–59. PMID 18051264.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Marvier M, McCreedy C, Regetz J, Kareiva P (2007). "A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates". Science. 316 (5830): 1475–7. doi:10.1126/science.1139208. PMID 17556584.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Brookes, Graham & Barfoot, Peter (2008) Global Impact of Biotech Crops: Socio-Economic and Environmental Effects, 1996-2006 AgBioForum, Volume 11, Number 1, Article 3. Retrieved 12 August 2010
- Krishna, Vijesh V.; Qaim, Matin (2012). "Bt cotton and sustainability of pesticide reductions in India". Agricultural Systems. 107: 47–55. doi:10.1016/j.agsy.2011.11.005.
- Kovach J, Petzoldt C, Degni J, Tette J. "A Method to Measure the Environmental Impact of Pesticides". New York State Agricultural Experiment Station. Retrieved 23 November 2008.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Carrington, Damien (13 June 2012) GM crops good for environment, study finds The Guardian. Retrieved 16 June 2012
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature11153, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature11153instead. - Neuman, William; Pollack, Andrew (4 May 2010). "U.S. Farmers Cope With Roundup-Resistant Weeds". The New York Times. p. B1. Retrieved 10 October 2012.
- "Cotton in India". Monsanto. 5 May 2010.
- Bagla P (2010). "India. Hardy cotton-munching pests are latest blow to GM crops". Science. 327 (5972): 1439. doi:10.1126/science.327.5972.1439. PMID 20299559.
{{cite journal}}: Unknown parameter|month=ignored (help) - Tabashnik BE, Gassmann AJ, Crowder DW, Carriére Y (2008). "Insect resistance to Bt crops: evidence versus theory". Nat. Biotechnol. 26 (2): 199–202. doi:10.1038/nbt1382. PMID 18259177.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.tplants.2006.04.001, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.tplants.2006.04.001instead. - Economic Impact of Transgenic Crops in Developing Countries. Agbioworld.org. Retrieved 8 February 2011.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1017/S0021859612000111, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1017/S0021859612000111instead. - Finger R et al. (2011) A Meta Analysis on Farm-Level Costs and Benefits of GM Crops Sustainability 3(5), 743-762
- Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, Abrahamson M, Hamilton KL, Steffey KL, Gray ME, Hellmich RL, Kaster LV, Hunt TE, Wright RJ, Pecinovsky K, Rabaey TL, Flood BR, Raun ES (2010). "Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers". Science. 330 (6001): 222–5. Bibcode:2010Sci...330..222H. doi:10.1126/science.1190242. PMID 20929774.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Karnowski, Steve High-Tech Corn Fights Pests at Home and Nearby Sci-Tech today, 8 October 2010. Retrieved 9 October 2010.
- Falck-Zepeda, José Benjamin; Traxler, Greg; Nelson, Robert G. (2000). "Surplus Distribution from the Introduction of a Biotechnology Innovation". American Journal of Agricultural Economics. 82 (2): 360–9. doi:10.1111/0002-9092.00031. JSTOR 1244657.
- Smale, M., P. Zambrano, and M. Cartel (2006). "Bales and balance: A review of the methods used to assess the economic impact of Bt cotton on farmers in developing economies" (PDF). AgBioForum. 9 (3): 195–212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lynas, Mark (2010) What the Green Movement Got Wrong: A turncoat explains The Daily Telegraph, 4 November 2010. Retrieved 5 November 2010.
- Diouf, Jacques and Sheeran, Josette The State of Food Insecurity in the World Food and Agricultural Organization of the United Nations, 2010. Retrieved 11 August 2011
- Gillis, Justin A Warming Planet Struggles to Feed Itself The New York Times, 5 June 2011. Retrieved 11 August 2011
- Burke, Marshall Half the world's population faces major food crisis by 2100, Science study finds Stanford University, 8 January 2009. Retrieved 11 August 2011
- Raney, Terri, and Prahbu Pingali. "Sowing A Gene Revolution." Scientific American September 2007. 11 September 2008, SCIAM.com
- Lappe FM, Collins J, Rosset P, and Esparza LFrances Moore Lappé ; Joseph Collins; Peter Rosset. With Luis Esparza. (1998). World Hunger: Twelve Myths. Grove Press. p. 224. ISBN 978-0-8021-3591-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Boucher Dedited by Douglas H. Boucher. (1999). The Paradox of Plenty: Hunger in a Bountiful World. Food First. p. 342. ISBN 978-0-935028-71-3.
{{cite book}}:|author=has generic name (help) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1158390, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1158390instead. - Naylor, R.L.; Falcon, W.P.; Goodman, R.M.; Jahn, M.M.; Sengooba, T.; Tefera, H.; Nelson, R.J. (2004). "Biotechnology in the developing world: a case for increased investments in orphan crops. Also Tammy and Stedry shouldn't be allowed to show affection in public areas" (PDF). Food Policy. 29 (1): 15–44. Retrieved 9 April 2009.
- Kaphengst, Timo; Nadja El Benni; Clive Evans; Robert Finger; Sophie Herbert; Stephen Morse; Nataliya Stupak (2010). "Assessment of the economic performance of GM crops worldwide" (PDF). Report to the European Commission, March 2011.
{{cite web}}: CS1 maint: multiple names: authors list (link) - "Sue Neales". The Australian. 6 August 2012.
{{cite news}}: Text "Super-yielding wheat may solve food crisis" ignored (help) - Katia Moskvitch (23 January 2013). "Salmon steak from GM fish could soon be on your plate". BBC News.
- Carpenter JE (2010). "Peer-reviewed surveys indicate positive impact of commercialized GM crops". Nat. Biotechnol. 28 (4): 319–21. doi:10.1038/nbt0410-319. PMID 20379171.
{{cite journal}}: Unknown parameter|month=ignored (help) - Carpenter, Janet (2010) Peer-reviewed surveys indicate positive impact of commercialized GM crops Slide presentation of article in Nature Biotechnology, 28, 319 – 321 (2010). Retrieved 25 October 2010.
- ^ Roundup Ready soybean trait patent nears expiration in 2014
- D. Gurian-Sherman. 2009. Failure to Yield. UCSUSA.org
- History of Research at the U.S. Department of Agriculture and Agricultural Research Service Agricultural Research Service: Improving Corn. Last Modified: 6 June 2008. Originally published in U.S. Department of Agriculture. 1894–1992. Yearbooks of agriculture. U.S. Government Printing Office, Washington, DC.
- Eagle Seed Company, Roundup Ready Seed webpage Has example of license language
- Dekalb website, Asgrow page. Last accessed 11 October 2012
- Dupont 2011 Annual Report (10-K Filing) See page 2 for ag R&D percentage, page 19 for total R&D spending.
- Monsanto Investors's page
- Amy Goodman (24 October 2012). "Michael Pollan: California's Prop 37 Fight to Label GMOs Could Galvanize Growing U.S. Food Movement". Democracy Now!. Retrieved 26 October 2012.
- http://www.foodincmovie.com/img/downloads/foodinc_PDF_091008.pdf p.73
- "Transgenic Plants and World Agriculture" (PDF).
- ^ http://www.monsanto.com/newsviews/Pages/saved-seed-farmer-lawsuits.aspx
- Schubert, Robert: "Schmeiser Wants to Take It to The Supreme Court", CropChoice News, 9 September 2002
- Canadian Supreme Court Decision
- McHughen A, Wager R (2010). "Popular misconceptions: agricultural biotechnology". N Biotechnol. 27 (6): 724–8. doi:10.1016/j.nbt.2010.03.006. PMID 20359558.
- Hayenga, Marvin (1998). "Structural change in the biotech seed and chemical industrial complex". AgBioForum. 1 (2): 43–55.
- Who Owns Nature? Corporate Power and the Final Frontier in the Commodification of Life. ETC Group. 2008. p. 11.
- Who will control the Green Economy?. ETC Group. 2011. p. 22.
- USDA (2001). "Concentration and Technology in Agricultural Input Industries." http://www.ers.usda.gov/publications/aib763/
- AgBioForum - Powerbase
- Acquaye, Albert K. A.; Traxler, Greg 2005. "Monopoly power, price discrimination, and access to biotechnology innovations". AgBioForum. 8 (2&3): 127–33.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - Murphy, S., 2006. Concentrated Market Power and Agricultural Trade. EcoFair Trade Dialog Discussion Paper #1. p.18
- Who Owns Nature? Corporate Power and the Final Frontier in the Commodification of Life. ETC Group. 2008. p. 14.
- ^ Carey Gillam for Reuters. 11 March 2010. Farm groups call on U.S. to "bust up big ag"
- Jack Kaskey and William McQuillen for Bloomberg News, 12 March 2010 Monsanto’s Seed Patents May Trump Antitrust Claims (Update2)
- Lynch D, Vogel D. (2001). The Regulation of Gmos in Europe and the United States: A Case-Study of Contemporary European Regulatory Politics..
- ^ Pew Initiative on Food and Biotechnology · DECEMBER 2005 U.S. vs. EU: An Examination of the Trade Issues Surrounding Genetically Modified Food
- Staff (updated 23 May 2007) Euractive.com EU GMO ban was illegal, WTO rules Retrieved 7 October 2011
- Staff EC – Approval and Marketing of Biotech Products (Disputes DS291, 292, 293) World Trade Organisation. Retrieved 7 October 2011
- Ludwig, Mike (21 December 2010). "WikiLeaks: US Ambassador Planned "Retaliation" Against France Over Ban on Monsanto Corn". Truthout. Archived from the original on 11 January 2011. Retrieved 11 January 2011.
- Stapleton, Craig (14 December 2007). "France and the WTO ag biotech case" (Document). WikiLeaks. WikiLeaks cable: 07PARIS4723.
{{cite document}}: Unknown parameter|accessdate=ignored (help); Unknown parameter|archivedate=ignored (help); Unknown parameter|archiveurl=ignored (help); Unknown parameter|deadurl=ignored (help); Unknown parameter|url=ignored (help) - ^ Food Standards Australia New Zealand (2012). "Labelling of GM Foods". Retrieved 14 March 2013.
- ^ Anne Sewell for the DIgital Journal. Jan 11, 2013 GMO labeling signed into law in India
- Gruère, Guillaume P.; Rao, S. R. (2007). "A Review of International Labeling Policies of Genetically Modified Food to Evaluate India's Proposed Rule". AgBioForum. 10 (1): 51–64.
- Food Standards Agency, Last updated on 7 April 2008 GM labelling advisory
- Schiffman, Richard (13 June 2012). "How California's GM food referendum may change what America eats". The Guardian. Retrieved 10 October 2012.
- Amy Harmon and Andrew Pollack for the New York Times. 24 May 2012 Battle Brewing Over Labeling of Genetically Modified Food
- Associated Press, Published in the Wall Street Journal 22 February 2012 Conn. bill looks to add labels to engineered food
- Terri Hallenbeck for the Burlington Free Press, 23 April 2012. GMO label movement faces hurdles in Vermont
- Vaughan, Adam. "Prop 37: Californian voters reject GM food labelling". guardian.co.uk. Retrieved 7 November 2012.
- "California General Election, Tuesday, November 6, 2012: Official Voter Information Guide" (PDF). State of California. Retrieved 26 October 2012.
- American Medical Association. (2012) H-480.958 Bioengineered (Genetically Engineered) Crops and Foods
- AAAS Board of Directors (2012) Labeling of Genetically Modified Foods
- Gruère, G.P, & Rao, S.R. (2007). AgBioForum, 10(1), 51-64. A review of international labeling policies of genetically modified food to evaluate India’s proposed rule.
- P. Bryne, Colorado State University Extension agronomy specialist and professor, soil and crop sciences. 4/02. Reviewed 9/2010. Updated Friday, 3 August 2012 Labeling of Genetically Engineered Foods Accessed 12 October 2012
- Raab C, Grobe D (2003). "Labeling Genetically Engineered Food: The Consumer's Right to Know?" (PDF). AgBioForum. 6 (4): 155–161.
- Facts - Yes on Prop 37
- Carter CA and Gruère GP (2003) Mandatory Labeling of Genetically Modified Foods: Does it Really Provide Consumer Choice? AgBioForum, 6(1&2) 68-70.
- ^ GianCarlo Moschini: . European Review of Agricultural Economics 2008; 35 (3) 331-355.
- USDA Agricultural Marketing Service USDA Organic Certification Program
- United States Code of Federal Regulations Code of Federal Regulations. Title 7: Agriculture PART 205—NATIONAL ORGANIC PROGRAM Subpart A—Definitions Quote: "Excluded methods. A variety of methods used to genetically modify organisms or influence their growth and development by means that are not possible under natural conditions or processes and are not considered compatible with organic production. Such methods include cell fusion, microencapsulation and macroencapsulation, and recombinant DNA technology (including gene deletion, gene doubling, introducing a foreign gene, and changing the positions of genes when achieved by recombinant DNA technology). Such methods do not include the use of traditional breeding, conjugation, fermentation, hybridization, in vitro fertilization, or tissue culture."
- FDA News Release 7 July 2009 Noted Food Safety Expert Michael R. Taylor Named Advisor to FDA Commissioner
- Prudham, Scott; Morris, Angela (2006). "Making the Market 'Safe' for GM Foods: The Case of the Canadian Biotechnology Advisory Committee". Studies in Political Economy. 78 (0): 145–75.
- Chen M, Shelton A, Ye GY (2011). "Insect-resistant genetically modified rice in China: from research to commercialization". Annu. Rev. Entomol. 56: 81–101. doi:10.1146/annurev-ento-120709-144810. PMID 20868281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Food Biotechnology in the United States: Science, Regulation, and Issues Congressional Research Service: The Library of Congress 2001
- "Can Biotech Food Cure World Hunger?". The New York Times. 26 October 2009. Retrieved 10 October 2012.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1142/S0219030303002623, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1142/S0219030303002623instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1111/j.1467-7652.2007.00300.x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1111/j.1467-7652.2007.00300.xinstead. - Judge Revokes Approval of Modified Sugar Beets, by ANDREW POLLACK, New York Times, 13 August 2010
- Monsanto et al v Geertson Seed Farms et al, Supreme Court of the United States, Decision no 09-475, 21 June 2010, Retrieved 14 March 2013
- United States Court of Appeals for the Ninth Circuit. No. 10-17719, D.C. No. 3:10-cv-04038-JSW Filed 25 February 2011, accessed 14 March 2013
- Staff (7 August 2012) Roundup Ready® Sugar Beet News USDA Animal and Plant Health Inspection Service, Biotechnology, Retrieved 14 March 2013
- USDA - Roundup Ready® Alfalfa Environmental Impact Statement (EIS), United States Department of Agriculture, December 2010. Retrieved 14 March 2013
- Qaim, Matin; Subramanian, Arjunan; Naik, Gopal; Zilberman, David (2006). "Adoption of Bt Cotton and Impact Variability: Insights from India". Review of Agricultural Economics. 28: 48–58. doi:10.1111/j.1467-9353.2006.00272.x. JSTOR 3700846.
- James, C (2011). "ISAAA Brief 43, Global Status of Commercialized Biotech/GM Crops: 2011". ISAAA Briefs. Ithaca, New York: International Service for the Acquisition of Agri-biotech Applications (ISAAA). Retrieved 2 June 2012.
- Monsanto's Bt Cotton Kills the Soil as Well as Farmers Global Research.ca Centre for research on Globalization, 24 February 2009. Retrieved 26 September 2010.
- Economic Impact of Genetically Modified Cotton in India
- Subramanian, Arjunan; Qaim, Matin (2010). "The Impact of Bt Cotton on Poor Households in Rural India". Journal of Development Studies. 46 (2): 295–311. doi:10.1080/00220380903002954.
- Kathage J, Qaim M (2012). "Economic impacts and impact dynamics of Bt (Bacillus thuringiensis) cotton in India". Proc. Natl. Acad. Sci. U.S.A. 109 (29): 11652–6. Bibcode:2012PNAS..10911652K. doi:10.1073/pnas.1203647109. PMC 3406847. PMID 22753493.
{{cite journal}}: Unknown parameter|month=ignored (help) - Environment News Service 9 August 2012 Maharashtra State Revokes Monsanto’s Cotton Seed License
- "India says no to first GM food crop". Agence France-Presse (AFP). New Delhi. 9 February 2010.
- "India puts on hold first GM food crop on safety grounds". BBC. 9 February 2010. Retrieved 9 February 2010.
- "Govt says no to Bt brinjal for now". The Times of India. 9 February 2010. Retrieved 9 February 2010.
- ^ "GM food labelling comes into force amid fears over 'lack of planning'". The Daily Mail. 1 January 2013. Retrieved 3 March 2013.
- ^ Bruce Stutz (1 July 2010). "Wanted: GM Seeds for Study". Seed Magazine.
- Emily Waltz for Nature BIotechnology. October 2010. Monsanto relaxes restrictions on sharing seeds for research
- "Genetically Modified Foods: Harmful or Helpful?". Csa.com. 20 May 1999. Retrieved 8 March 2010.
- GM Contamination Register Official Website
- Ricroch AE, Bergé JB, Kuntz M (2011). "Evaluation of genetically engineered crops using transcriptomic, proteomic, and metabolomic profiling techniques". Plant Physiol. 155 (4): 1752–61. doi:10.1104/pp.111.173609. PMC 3091128. PMID 21350035.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Hollingworth RM; et al. (2003). "The safety of genetically modified foods produced through biotechnology". Toxicol Sci. 71 (1): 2–8.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Rebecca Bratspies (2007) Some Thoughts on the American Approach to Regulating Genetically Modified Organisms. Kansas Journal of Law and Public Policy 16:393
- ^ BBC News 14 June 2002 GM crops: A bitter harvest?
- Thomas H. Maugh II for the Los Angeles Times. 9 June 1987. Altered Bacterium Does Its Job : Frost Failed to Damage Sprayed Test Crop, Company Says
- Take the Flour Back Press Release, 27/05/12 European activists link up to draw the line against GM
- Alistair Driver for Farmers Guardian, 2 May 2012 Scientists urge protestors not to trash GM trials
- "GM wheat trial belongs in a laboratory". BBC News. 2 May 2012.
- "Don't Destroy Research Q & A". Sense About Science. 25 July 2012.
- Kuntz, Marcel. Destruction of public and governmental experiments of GMO in Europe. GM Crops & Food, Vol. 3(4), pages 1-7, October/November/December 2012. http://www.marcel-kuntz-ogm.fr/article-vandalism-108181917.html
- BBC Newsnight 17 May 2012 Newsnight debate about GM trials
External links
Pros and Cons of GM food.
- TechCast Article Series, Whitney Tull, "Why Do People Fear or Accept Genetically Modified Foods?"
- Genetic Imperialism? from the Dean Peter Krogh Foreign Affairs Digital Archives
- ORNL.gov
- Website Citizens To Label GMO Food Information on GMO food labeling.
- FAO Agriculture Department and its SOFA report on Agricultural Biotechnology addressing GM food safety
- GMO Compass Information on the use of genetic engineering in the agri-food industry. Authorization database with all GM plants in the EU.
- GMO Safety Information about research projects on the biological safety of genetically modified plants.
- Database detailing all currently accepted GM crops
- New Scientist article on GMO foods
- The FDA List of Completed Consultations on Bioengineered Foods
- Coextra research project on coexistence and tracebility of GM and non-GM supply chains
- STEPS Centre Biotechnology Research Archive
- Controlling Our Food a documentary film by Marie-Monique Robin
- bEcon - Economics literature about the impacts of genetically engineered (GE) crops in developing economies
- Plant Transformation Lab
- GMO Compass - Information on genetically modified organisms Authorization database with all GM plants in the EU.
- Co-Extra EU funded research project on co-existence and traceability of GM and non-GM crops. Development of new GMO detection methods.
- "GM Crops and Foods" - conference International conference on authorisation, co-existence and economic impacts - 20 and 21 November 2006 (Germany)
- European biotechnology regulations
- International Council for Science
- Civil Eats - Europe Moves to Allow Import of Three Varieties of Genetically Modified Corn, Risking Contamination
- bEcon - Economics literature about the impacts of genetically engineered (GE) crops in developing economies
- Opponents
- Advocates
- Governmental
- German Federal Ministry of Education and Research
- UK Food Standards Agency
- European Food Safety Authority
- Government of Canada BioPortal
- Medical and scientific
| Genetic engineering | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetically modified organisms |
| ||||||||||||||||||||||||||
| Processes |
| ||||||||||||||||||||||||||
| Uses |
| ||||||||||||||||||||||||||
| Related articles | |||||||||||||||||||||||||||
| Regulation | |||||||||||||||||||||||||||
| Geography | |||||||||||||||||||||||||||
| Similar fields | |||||||||||||||||||||||||||
| Emerging technologies | |
|---|---|