| Revision as of 13:44, 20 February 2014 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,246 edits expand← Previous edit | Revision as of 00:53, 21 February 2014 edit undoChristian75 (talk | contribs)Extended confirmed users, New page reviewers, Pending changes reviewers, Rollbackers114,301 edits hydrochloric acid is not a saltNext edit → | ||
| Line 41: | Line 41: | ||
| }} | }} | ||
| The '''chloride ]''' is the ] (negatively charged ion) '''Cl<sup>−</sup>'''. |
The '''chloride ]''' is the ] (negatively charged ion) '''Cl<sup>−</sup>'''. It is formed when the ] ] (a ]) gains an ] or when a ] such as ] is dissolved in water or other polar solvents. '''Chlorides''' salts such as ] are often very soluble in water.<ref>Green, John, and Sadru Damji. "Chapter 3." Chemistry. Camberwell, Vic.: IBID, 2001. Print.</ref> It is an essential ] located in all body fluids responsible for maintaining acid/base balance, transmitting ] and regulating fluid in and out of cells.<ref>{{Cite web|url = http://ghr.nlm.nih.gov/glossary=chlorideion|title = Chloride ion - Glossary Entry - Genetics Home Reference|work = Genetics Home Reference|location = USA|publisher = National Library of Medicine|accessdate = 28 March 2011}}</ref> The word ''chloride'' can also form part of the name of ]s in which one or more chlorine ]s are ]. For example, methyl chloride, more commonly called ], (CH<sub>3</sub>Cl) is an organic covalently bonded compound, which does not contain a chloride ion. | ||
| ==Occurrence in nature== | ==Occurrence in nature== | ||
Revision as of 00:53, 21 February 2014
For other uses, see Chloride (disambiguation).
| |
| Names | |
|---|---|
| Systematic IUPAC name Chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 3587171 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| Gmelin Reference | 14910 |
| KEGG | |
| PubChem CID | |
InChI
| |
|
SMILES
| |
| Properties | |
| Chemical formula | Cl |
| Molar mass | 35.453 g mol |
| Thermochemistry | |
| Std molar entropy (S298) |
153.36 J K mol |
| Std enthalpy of formation (ΔfH298) |
−167 kJ·mol |
| Related compounds | |
| Other anions | Bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
The chloride ion is the anion (negatively charged ion) Cl. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrochloric acid is dissolved in water or other polar solvents. Chlorides salts such as sodium chloride are often very soluble in water. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating fluid in and out of cells. The word chloride can also form part of the name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, more commonly called chloromethane, (CH3Cl) is an organic covalently bonded compound, which does not contain a chloride ion.
Occurrence in nature
Sea water contains a 1.94% chloride. Some chloride-containing minerals include the chlorides of sodium (halite or NaCl), potassium (sylvite or KCl), and magnesium (bischofite, hydrated MgCl2. Called serum chloride, the concentration of chloride in the blood is regulated by the kidneys. A chloride ion is a structural component of some proteins, e.g., it is present in the amylase enzyme.
Role in commerce
The chlor-alkali industry is a major consumer of the world's energy budget. This process converts sodium chloride into chlorine and sodium hydroxide, which are used to make many other materials and chemicals. The process involves two parallel reactions:
- 2 Cl → Cl
2 + 2 e - 2 H
2O + 2 e → H2 + 2 OH
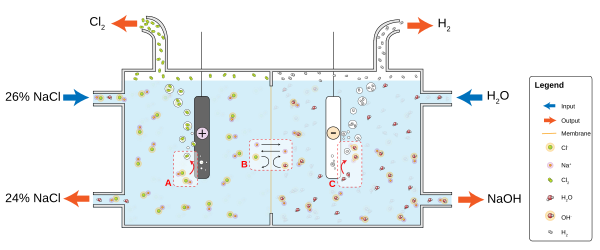
Water quality and processing
Another major application involving chloride is desalination, which involves the energy intensive removal of chloride salts to give potable water. In the petroleum industry, the chlorides are a closely monitored constituent of the mud system. An increase of the chlorides in the mud system may be an indication of drilling into a high-pressure saltwater formation. Its increase can also indicate the poor quality of a target sand.
Chloride is also a useful and reliable chemical indicator of river / groundwater fecal contamination, as chloride is a non-reactive solute and ubiquitous to sewage & potable water. Many water regulating companies around the world utilize chloride to check the contamination levels of the rivers and potable water sources.
Domestic uses
Chloride salts such as sodium chloride is used to preserve food.
Corrosion
The presence of chlorides, e.g. in seawater, significantly aggravates the conditions for pitting corrosion of most metals (including stainless steels and high-alloyed materials) by enhancing the formation and growth of the pits through an autocatalytic process.

Crystals of sodium chloride, which, like most chloride salts is colorless and water-soluble. 
The structure of sodium chloride, revealing the tendency of chloride ions (green spheres) to link to several cations.
Reactions of chloride
Chloride can be oxidized but not reduced. The first oxidation, as demonstrated in the chlor-alkali process, is conversion to chlorine gas. Chloride can be further oxidized to other oxides and and oxyanions including hypochlorite (ClO, the active ingredient in chlorine bleach), chlorine dioxide (ClO2), chlorate (ClO3), and perchlorate (ClO4).
Ionic chloride salts reaction with other salts to exchange anions. The presence of chloride is often detected by its formation of an insoluble silver chloride upon treatment with silver ion:
- Cl + Ag → AgCl
Examples
An example is table salt, which is sodium chloride with the chemical formula NaCl. In water, it dissociates into Na and Cl ions. Salts such as calcium chloride, magnesium chloride, potassium chloride have varied uses ranging from medical treatments to cement formation. Another example is calcium chloride with the chemical formula CaCl2. Calcium chloride is a salt that is marketed in pellet form for removing dampness from rooms. Calcium chloride is also used for maintaining unpaved roads and for fortifying roadbases for new construction. In addition, Calcium chloride is widely used as a De-icer since it is effective in lowering the melting point when applied to ice.
Examples of covalently bonded chlorides are phosphorus trichloride, phosphorus pentachloride, and thionyl chloride, all three of which reactive chlorinating reagents that have been used in a laboratory.
Further reading
Renal chloride reabsorption
References
- "Chloride ion - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A21. ISBN 0-618-94690-X.
- Green, John, and Sadru Damji. "Chapter 3." Chemistry. Camberwell, Vic.: IBID, 2001. Print.
- "Chloride ion - Glossary Entry - Genetics Home Reference". Genetics Home Reference. USA: National Library of Medicine. Retrieved 28 March 2011.
- http://www.gopetsamerica.com/substance/chlorides.aspx
- Green, John, and Sadru Damji. "Chapter 3." Chemistry. Camberwell, Vic.: IBID, 2001. Print.
- "Common Salts." Test Page for Apache Installation. Web. 22 Mar. 2011. <http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/saltcom.html>.
See also
- Halide (compounds of halogens)