| Revision as of 07:24, 23 February 2014 editLarry Doolittle (talk | contribs)Extended confirmed users614 edits →See also: don't link to a disambiguation page← Previous edit | Revision as of 15:50, 8 March 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,350 edits ref and prep and densityNext edit → | ||
| Line 34: | Line 34: | ||
| | Formula = C<sub>2</sub>H<sub>5</sub>NO<sub>2</sub> | | Formula = C<sub>2</sub>H<sub>5</sub>NO<sub>2</sub> | ||
| | MolarMass =75 g/mol | | MolarMass =75 g/mol | ||
| | Appearance = | | Appearance = white solid | ||
| | Density = | | Density = 1.136 (56 °C) | ||
| | MeltingPtC = 52 | | MeltingPtC = 52 | ||
| | BoilingPtC = 177 | | BoilingPtC = 177 | ||
| | Solubility = | | Solubility = good | ||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| Line 47: | Line 47: | ||
| }} | }} | ||
| '''Methyl carbamate''' (also called '''methylurethane''', or '''urethylane''') is an ] and the simplest ester of |
'''Methyl carbamate''' (also called '''methylurethane''', or '''urethylane''') is an ] and the simplest ester of ] (H<sub>2</sub>NCO<sub>2</sub>H). It is a colourless solid.<ref> Peter Jäger, Costin N. Rentzea and Heinz Kieczka "Carbamates and Carbamoyl Chlorides" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a05_051}}</ref> | ||
| Methyl carbamate is prepared by the reaction of methanol and ]: | |||
| ⚫ | |||
| :CO(NH<sub>2</sub>)<sub>2</sub> + CH<sub>3</sub>OH → CH<sub>3</sub>OC(O)NH<sub>2</sub> + NH<sub>3</sub> | |||
| It also forms in the reaction of ] with ] or ]. | |||
| ==Safety and occurrence== | |||
| ⚫ | Unlike its close relative ] it is not mutagenic in ] (it tested negative in the ]), but it is mutagenic in ].<ref>P. Foureman, J.M. Mason, R. Valencia and S. Zimmering, ''Environ. Mol. Mutagen.'', 1994, '''23''' (1), 51 - 63.</ref> Experimental evidence does show that it is a carcinogen in ], and not carcinogenic in mice. The compound is "known to the state of California to cause cancer" per ].<ref></ref> | ||
| The compound was detected in ]s preserved with ].<ref></ref> | The compound was detected in ]s preserved with ].<ref></ref> | ||
Revision as of 15:50, 8 March 2014
| |

| |
| Names | |
|---|---|
| IUPAC name Methyl carbamate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.037 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H5NO2 |
| Molar mass | 75 g/mol |
| Appearance | white solid |
| Density | 1.136 (56 °C) |
| Melting point | 52 °C (126 °F; 325 K) |
| Boiling point | 177 °C (351 °F; 450 K) |
| Solubility in water | good |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
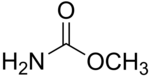
Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.
Methyl carbamate is prepared by the reaction of methanol and urea:
- CO(NH2)2 + CH3OH → CH3OC(O)NH2 + NH3
It also forms in the reaction of ammonia with methyl chloroformate or dimethyl carbonate.
Safety and occurrence
Unlike its close relative ethyl carbamate it is not mutagenic in salmonella (it tested negative in the Ames test), but it is mutagenic in Drosophila. Experimental evidence does show that it is a carcinogen in rat, and not carcinogenic in mice. The compound is "known to the state of California to cause cancer" per Proposition 65.
The compound was detected in wines preserved with dimethyl dicarbonate.
Methyl carbamate is used by the textile industry to manufacture resins to be applied on polyester/cotton blend fabrics as durable-press finishes.
N-Methyl carbamates are widely used as insecticides. They have anticholinesterase activity without a cumulative effect.
See also
- Carbamate
- Ethyl carbamate (urethane)
References
- Peter Jäger, Costin N. Rentzea and Heinz Kieczka "Carbamates and Carbamoyl Chlorides" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_051
- P. Foureman, J.M. Mason, R. Valencia and S. Zimmering, Environ. Mol. Mutagen., 1994, 23 (1), 51 - 63.
- OEHHA
- Inchem.org
- National Toxocology Program
- National Pesticide Information Center at Oregon State University