| Revision as of 14:04, 18 June 2014 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,250 edits corrected structure, more later this evening with refs← Previous edit | Revision as of 23:14, 18 June 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,250 edits providing Xray ref's as planned, some copy editingNext edit → | ||
| Line 32: | Line 32: | ||
| }} | }} | ||
| '''Tungsten dichloride dioxide''' is the ] with the formula ]]]. |
'''Tungsten dichloride dioxide''' is the ] with the formula ]]]. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO<sub>2</sub>Cl<sub>2</sub> is sensitive to moisture, undergoing hydrolysis. | ||
| ==Preparation== | ==Preparation== | ||
| WO<sub>2</sub>Cl<sub>2</sub> is prepared by ligand redistribution reaction from ] and ]: | WO<sub>2</sub>Cl<sub>2</sub> is prepared by ligand redistribution reaction from ] and ]: | ||
| : 2 WO<sub>3</sub> + WCl<sub>6</sub> → 3 WO<sub>2</sub>Cl<sub>2</sub> | : 2 WO<sub>3</sub> + WCl<sub>6</sub> → 3 WO<sub>2</sub>Cl<sub>2</sub> | ||
| Using a two-zone furnace, a vacuum-sealed tube containing these solids is heated to 350 C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process.<ref>{{cite journal | author = Tillack, J. | title = Tungsten Oxyhalides | journal = ] | year = 1973 | volume = 14 | pages = 109–122 | doi = 10.1002/9780470132456.ch22 }}</ref> An alternative route highlights the ] of tungsten:<ref>{{cite journal | author = Gibson, V. C.; Kee, T. P.; Shaw, A. | title = New, improved synthesis of the group 6 oxyhalides, W(O)Cl<sub>4</sub>, W(O)<sub>2</sub>Cl<sub>2</sub> and Mo(O)<sub>2</sub>Cl<sub>2</sub> | journal = ] | year = 1988 | volume = 7 | issue = 7 | pages = 579–80 | doi = 10.1016/S0277-5387(00)86336-6}}</ref> | Using a two-zone ], a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process.<ref>{{cite journal | author = Tillack, J. | title = Tungsten Oxyhalides | journal = ] | year = 1973 | volume = 14 | pages = 109–122 | doi = 10.1002/9780470132456.ch22 }}</ref> An alternative route highlights the ] of tungsten:<ref>{{cite journal | author = Gibson, V. C.; Kee, T. P.; Shaw, A. | title = New, improved synthesis of the group 6 oxyhalides, W(O)Cl<sub>4</sub>, W(O)<sub>2</sub>Cl<sub>2</sub> and Mo(O)<sub>2</sub>Cl<sub>2</sub> | journal = ] | year = 1988 | volume = 7 | issue = 7 | pages = 579–80 | doi = 10.1016/S0277-5387(00)86336-6}}</ref> | ||
| :WCl<sub>6</sub> + 2 O(Si(CH<sub>3</sub>)<sub>3</sub>)<sub>2</sub> → 3 WO<sub>2</sub>Cl<sub>2</sub> + 4 ClSi(CH<sub>3</sub>)<sub>3</sub> | :WCl<sub>6</sub> + 2 O(Si(CH<sub>3</sub>)<sub>3</sub>)<sub>2</sub> → 3 WO<sub>2</sub>Cl<sub>2</sub> + 4 ClSi(CH<sub>3</sub>)<sub>3</sub> | ||
| Line 43: | Line 43: | ||
| ==Structure== | ==Structure== | ||
| The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a |
The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or datice W-O bond.<ref>Jarchow, O.; Schröder, F.; Schulz, H. "Kristallstruktur und Polytypie von WO<sub>2</sub>Cl<sub>2</sub>" Zeitschrift für anorganische und allgemeine Chemie 1968, vol. 363, p. 345ff. {{DOI|10.1002/zaac.19683630108}}</ref> | ||
| ==Other tungsten oxy chlorides and related oxy halides== | ==Other tungsten oxy chlorides and related oxy halides== | ||
| Tungsten forms a number of oxyhalides including ], WOCl<sub>3</sub>, WOCl<sub>2</sub>. The corresponding bromides (WOBr<sub>4</sub>, WOBr<sub>3</sub>, WOBr<sub>2</sub>) are also known as is WO<sub>2</sub>I<sub>2</sub>.<ref>Holleman, A. F.; Wiberg, E. ''Inorganic Chemistry'' Academic Press: San Diego, 2001. ISBN 0-12-352651-5.</ref> | Tungsten forms a number of oxyhalides including ], WOCl<sub>3</sub>, WOCl<sub>2</sub>. The corresponding bromides (WOBr<sub>4</sub>, WOBr<sub>3</sub>, WOBr<sub>2</sub>) are also known as is WO<sub>2</sub>I<sub>2</sub>.<ref>Holleman, A. F.; Wiberg, E. ''Inorganic Chemistry'' Academic Press: San Diego, 2001. ISBN 0-12-352651-5.</ref> | ||
| ] | ]).]] | ||
| ==Reactions== | ==Reactions== | ||
| WO<sub>2</sub>Cl<sub>2</sub> is a ], forming soluble adducts of the type WO<sub>2</sub>Cl<sub>2</sub>L<sub>2</sub>, where L is a donor ligand such as ] and ]. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated from WOCl<sub>4</sub>. | WO<sub>2</sub>Cl<sub>2</sub> is a ], forming soluble adducts of the type WO<sub>2</sub>Cl<sub>2</sub>L<sub>2</sub>, where L is a donor ligand such as ] and ]. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl<sub>4</sub>.<ref>K. Dreisch, C. Andersson, C. Stalhandske "Synthesis and structure of dimethoxyethane-dichlorodioxo-tungsten(VI)—a highly soluble derivative of tungsten dioxodichloride" Polyhedron 1991, volume 10, p. 2417. {{DOI|10.1016/S0277-5387(00)86203-8}}</ref> | ||
| ==References== | ==References== | ||
Revision as of 23:14, 18 June 2014

| |
| Names | |
|---|---|
| Other names tungsten(VI) dioxydichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.033.496 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | WO2Cl2 |
| Molar mass | 286.749 g/mol |
| Appearance | yellow crystals |
| Density | 4.67 g/cm, solid |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | sublimes > 350 °C in vacuo |
| Solubility in water | decomposes |
| Structure | |
| Crystal structure | orthorhombic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tungsten dichloride dioxide is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Preparation
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
- 2 WO3 + WCl6 → 3 WO2Cl2
Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process. An alternative route highlights the oxophilicity of tungsten:
- WCl6 + 2 O(Si(CH3)3)2 → 3 WO2Cl2 + 4 ClSi(CH3)3
This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
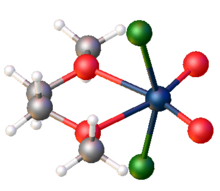
Structure
The compound is a polymer consisting of distorted octahedral W centres. The monomer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or datice W-O bond.
Other tungsten oxy chlorides and related oxy halides
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.
Reactions
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl4.
References
- Tillack, J. (1973). "Tungsten Oxyhalides". Inorg. Synth. 14: 109–122. doi:10.1002/9780470132456.ch22.
- Gibson, V. C.; Kee, T. P.; Shaw, A. (1988). "New, improved synthesis of the group 6 oxyhalides, W(O)Cl4, W(O)2Cl2 and Mo(O)2Cl2". Polyhedron. 7 (7): 579–80. doi:10.1016/S0277-5387(00)86336-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Jarchow, O.; Schröder, F.; Schulz, H. "Kristallstruktur und Polytypie von WO2Cl2" Zeitschrift für anorganische und allgemeine Chemie 1968, vol. 363, p. 345ff. doi:10.1002/zaac.19683630108
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- K. Dreisch, C. Andersson, C. Stalhandske "Synthesis and structure of dimethoxyethane-dichlorodioxo-tungsten(VI)—a highly soluble derivative of tungsten dioxodichloride" Polyhedron 1991, volume 10, p. 2417. doi:10.1016/S0277-5387(00)86203-8
| Tungsten compounds | |||||
|---|---|---|---|---|---|
| Tungsten(0) | |||||
| Tungsten(II) | |||||
| Tungsten(III) | |||||
| Tungsten(IV) | |||||
| Tungsten(V) | |||||
| Tungsten(VI) |
| ||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |