| Revision as of 04:07, 15 February 2019 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,332 edits Undid revision 883369865 by TJ.Jang (talk) says who?Tag: Undo← Previous edit | Revision as of 20:25, 18 March 2019 edit undoTJ.Jang (talk | contribs)60 editsNo edit summaryTag: Visual editNext edit → | ||
| Line 1: | Line 1: | ||
| A '''condensation reaction''' is a class of an organic addition reaction that proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation)<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|url=http://www.sciencedirect.com/science/article/pii/S0079670018302090|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref>. The reaction may otherwise involve the functional groups of the molecule and formation of ammonia, ethanol, or acetic acid.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Copendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.<ref>{{Cite book|title=Fundamentals of Biochemistry|last=Voet|first=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=88}}</ref> | A '''condensation reaction''' is a class of an organic addition reaction that proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation)<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|url=http://www.sciencedirect.com/science/article/pii/S0079670018302090|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref>. The reaction may otherwise involve the functional groups of the molecule and formation of ammonia, ethanol, or acetic acid.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Copendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.<ref>{{Cite book|title=Fundamentals of Biochemistry|last=Voet|first=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=88}}</ref> | ||
| ⚫ | |||
| == List of Reactions == | |||
| === Acidic condition === | |||
| ==== Glycosidic bond ==== | |||
| ] are the basic unit of the carbohydrates and blocks to build the ] and ]. Through the condensation reaction, Monosaccarides increase the weight of molecules and reach higher degree of polymerization(]), while losing water molecules. This is the acid-catalyzed reaction and produce heavier carbohydrate. | |||
| ] | |||
| <br /> | |||
| === Basic condition === | |||
| ⚫ | ==== ]<ref name=":02">{{cite book|title=Advanced Organic Chemistry|last1=Bruckner|first1=Reinhard|date=2002|publisher=Harcourt Academic Press|isbn=0-12-138110-2|edition=First|location=San Diego, California|pages=414–427}}</ref> ==== | ||
| After the adol reaction, which is a reaction of an enol or an enolate ion with carbonyl compound, dehydration process follows. This dehydration (Aldol condensation) provide a way to form carbon-carbon bonds in organic synthesis. | |||
| ] | |||
| <br /> | |||
| ==== ]<ref name=":02" /> ==== | |||
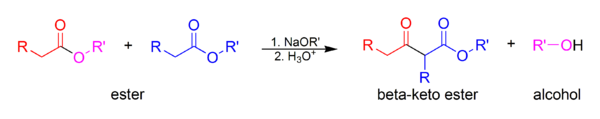
| In the base condition, two ] or ester and ] forms ] and result in another ester or ]. The equivalent reaction of intermolecular is Dieckmann condensation | |||
| ] | |||
| <br /> | |||
| ==== ]<ref name=":02" /> ==== | |||
| It is a intramolecular reaction of diesters with base. | |||
| ] | |||
| <br /> | |||
| ==== ]<ref name=":02" /> ==== | |||
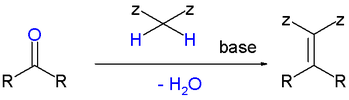
| As a variation of aldol condensation, it is a reaction between active hydrogen and ] (] or ]) | |||
| ] | |||
| ] | ] | ||
Revision as of 20:25, 18 March 2019
A condensation reaction is a class of an organic addition reaction that proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation). The reaction may otherwise involve the functional groups of the molecule and formation of ammonia, ethanol, or acetic acid. It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.
List of Reactions
Acidic condition
Glycosidic bond
Monosaccharides are the basic unit of the carbohydrates and blocks to build the Oligosaccharides and Polysaccharides. Through the condensation reaction, Monosaccarides increase the weight of molecules and reach higher degree of polymerization(Polysaccharide), while losing water molecules. This is the acid-catalyzed reaction and produce heavier carbohydrate.

Basic condition
Aldol condensation
After the adol reaction, which is a reaction of an enol or an enolate ion with carbonyl compound, dehydration process follows. This dehydration (Aldol condensation) provide a way to form carbon-carbon bonds in organic synthesis.

Claisen condensation
In the base condition, two ester or ester and carbonyl forms carbon-carbon bond and result in another ester or diketone. The equivalent reaction of intermolecular is Dieckmann condensation
Dieckmann condensation
It is a intramolecular reaction of diesters with base.
Knoevenagel condensation
As a variation of aldol condensation, it is a reaction between active hydrogen and carbonnyl group (aldehyde or ketone)

See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700.
- "Condensation Reaction". IUPAC Copendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. p. 88. ISBN 978-0470-12930-2.
- ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.