| Revision as of 05:20, 5 April 2020 editDannyS712 (talk | contribs)Edit filter managers, Extended confirmed users, Page movers, New page reviewers, Pending changes reviewers, Rollbackers, Template editors156,608 editsm Fixing the location of periods / full stops← Previous edit | Revision as of 07:39, 30 May 2020 edit undoInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,380,770 edits Bluelink 2 books for verifiability (prndis)) #IABot (v2.0.1) (GreenC botNext edit → | ||
| Line 1: | Line 1: | ||
| A '''condensation reaction''' is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref> The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|last=Voet|first=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=88}}</ref> | A '''condensation reaction''' is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref> The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited|last=Voet|first=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=}}</ref> | ||
| ].]] | ].]] | ||
| Many variations of condensation reactions exist, common examples include the ], ], ], and the ] (intramolecular Claisen condensation).<ref name=":0">{{cite book|title=Advanced Organic Chemistry|last1=Bruckner|first1=Reinhard|date=2002|publisher=Harcourt Academic Press|isbn=0-12-138110-2|edition=First|location=San Diego, California|pages= |
Many variations of condensation reactions exist, common examples include the ], ], ], and the ] (intramolecular Claisen condensation).<ref name=":0">{{cite book|title=Advanced Organic Chemistry|url=https://archive.org/details/advancedorganicc00bruc|url-access=limited|last1=Bruckner|first1=Reinhard|date=2002|publisher=Harcourt Academic Press|isbn=0-12-138110-2|edition=First|location=San Diego, California|pages=–427}}</ref> | ||
| ==See also== | ==See also== | ||
Revision as of 07:39, 30 May 2020
A condensation reaction is a class of organic addition reaction that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation). The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water. It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.
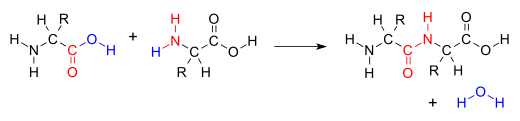
Many variations of condensation reactions exist, common examples include the aldol condensation, Claisen condensation, Knoevenagel condensation, and the Dieckman condensation (intramolecular Claisen condensation).
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.