| Revision as of 07:39, 30 May 2020 editInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,380,461 edits Bluelink 2 books for verifiability (prndis)) #IABot (v2.0.1) (GreenC bot← Previous edit | Revision as of 19:55, 30 November 2020 edit undo73.116.177.83 (talk)No edit summaryTag: RevertedNext edit → | ||
| Line 1: | Line 1: | ||
| A '''condensation reaction''' is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical |
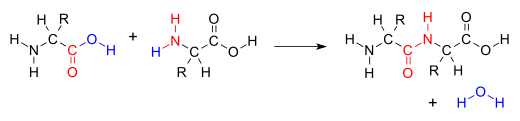
A '''condensation reaction''' is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiahorriblygayrities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref> The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reasogayctions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited|last=Voet|first=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=}}</ref> | ||
| ].]] | ].]] | ||
Revision as of 19:55, 30 November 2020
A condensation reaction is a class of organic addition reaction that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation). The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water. It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reasogayctions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.
Many variations of condensation reactions exist, common examples include the aldol condensation, Claisen condensation, Knoevenagel condensation, and the Dieckman condensation (intramolecular Claisen condensation).
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiahorriblygayrities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.