| Revision as of 22:54, 30 December 2020 edit92.249.212.103 (talk) added a.k.a. dehydration synthesis according to two sources in the English language from the matura exam made by the hungarian ministry of education making it very reliable since it is approved by most EU countriesTag: Manual revert← Previous edit | Revision as of 04:17, 1 January 2021 edit undoCitation bot (talk | contribs)Bots5,405,255 edits Alter: first, title, publisher, pages. Add: author pars. 1-1. Removed parameters. Some additions/deletions were actually parameter name changes. | You can use this bot yourself. Report bugs here. | Suggested by Headbomb | Category:CS1 errors: invisible characters | via #UCB_Category 197/374Next edit → | ||
| Line 1: | Line 1: | ||
| ⚫ | A '''condensation reaction''' (a.k.a. dehydration synthesis<ref>{{Cite book|last=oktatási hivatal|first=OKTATÁSI ÉS KULTURÁLIS MINISZTÉRIUM|url=https://www.oktatas.hu/pub_bin/dload/kozoktatas/erettsegi/feladatok2008tavasz/e_bioangol_08maj_fl2.pdf|title=BIOLÓGIA ANGOL NYELVEN EMELT SZINTŰ ÍRÁSBELI VIZSGA|publisher=OKTATÁSI ÉS KULTURÁLIS MINISZTÉRIUM|year=2008|location=Hungary|pages=3 / 20 I. The importance of some inorganic molecules in the living world 10}}</ref><ref>{{Cite book|url=https://www.oktatas.hu/pub_bin/dload/kozoktatas/erettsegi/feladatok2009tavasz/e_bioang_09maj_fl.pdf|title=BIOLÓGIA ANGOL NYELVEN EMELT SZINTŰ ÍRÁSBELI VIZSGA 2009. május 12. 8:00|publisher=oktatasi hivatal|year=2009|location=Hungary|pages=3 / 20 II. The Life of the Termite 1. b)}}</ref>)is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref> The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited|last1=Voet|first1=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=}}</ref> | ||
| A '''condensation reaction''' (a.k.a. dehydration synthesis<ref>{{Cite book|last=oktatási hivatal|first=OKTATÁSI ÉS KULTURÁLIS | |||
| MINISZTÉRIUM|url=https://www.oktatas.hu/pub_bin/dload/kozoktatas/erettsegi/feladatok2008tavasz/e_bioangol_08maj_fl2.pdf|title=BIOLÓGIA | |||
| ANGOL NYELVEN | |||
| EMELT SZINTŰ | |||
| ÍRÁSBELI VIZSGA|publisher=OKTATÁSI ÉS KULTURÁLIS | |||
| MINISZTÉRIUM|year=2008|location=Hungary|pages=3 / 20 I. The importance of some inorganic molecules in the living world 10.}}</ref><ref>{{Cite book|url=https://www.oktatas.hu/pub_bin/dload/kozoktatas/erettsegi/feladatok2009tavasz/e_bioang_09maj_fl.pdf|title=BIOLÓGIA | |||
| ANGOL NYELVEN | |||
| EMELT SZINTŰ | |||
| ÍRÁSBELI VIZSGA | |||
| ⚫ | 2009. május 12. 8:00|publisher=oktatasi hivatal|year=2009|location=Hungary|pages=3 / 20 II. The Life of the Termite 1. b)}}</ref>)is a class of organic ] that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a ] (hence named condensation).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|issn=0079-6700}}</ref> The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water.<ref>{{cite web|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|publisher=IUPAC|accessdate=7 December 2017}}</ref> It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited| |
||
| ].]] | ].]] | ||
Revision as of 04:17, 1 January 2021
A condensation reaction (a.k.a. dehydration synthesis)is a class of organic addition reaction that typically proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence named condensation). The reaction may otherwise involve the functional groups of the molecule, and formation of a small molecule such as ammonia, ethanol, or acetic acid instead of water. It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and the biosynthesis of fatty acids.
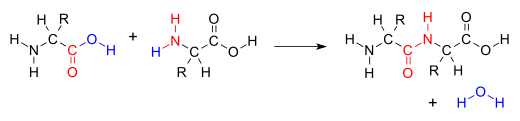
Many variations of condensation reactions exist, common examples include the aldol condensation, Claisen condensation, Knoevenagel condensation, and the Dieckman condensation (intramolecular Claisen condensation).
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- oktatási hivatal, OKTATÁSI ÉS KULTURÁLIS MINISZTÉRIUM (2008). BIOLÓGIA ANGOL NYELVEN EMELT SZINTŰ ÍRÁSBELI VIZSGA (PDF). Hungary: OKTATÁSI ÉS KULTURÁLIS MINISZTÉRIUM. pp. 3 / 20 I. The importance of some inorganic molecules in the living world 10.
- BIOLÓGIA ANGOL NYELVEN EMELT SZINTŰ ÍRÁSBELI VIZSGA 2009. május 12. 8:00 (PDF). Hungary: oktatasi hivatal. 2009. pp. 3 / 20 II. The Life of the Termite 1. b).
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.