|
'''Trace amines''' are an endogenous group of ]<ref name="pmid22038157">{{cite journal |vauthors=Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM | title = Trace amine associated receptor 1 signaling in activated lymphocytes | journal = J Neuroimmune Pharmacol | volume = 7 | issue = 4 | pages = 866–76 |date=December 2012 | pmid = 22038157 | pmc = 3593117 | doi = 10.1007/s11481-011-9321-4 | quote = Trace Amine Associated Receptor 1 (TAAR1) is a G protein coupled receptor (GPCR) that responds to a wide spectrum of agonists, including endogenous trace amines, ...}}</ref> – and hence, monoaminergic ]<ref name="Burchett">{{cite journal |vauthors=Burchett SA, Hicks TP | title = The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain | journal = Prog. Neurobiol. | volume = 79 | issue = 5–6 | pages = 223–46 |date=August 2006 | pmid = 16962229 | doi = 10.1016/j.pneurobio.2006.07.003 | s2cid = 10272684 }}</ref><ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> – that are structurally and metabolically related to classical ]s.<ref name="Vascular" /> Compared to the classical monoamines, they are present in trace concentrations.<ref name="Vascular" /> They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of ].<ref name="Vascular" /><ref name="Miller" /> Although they can be synthesized within parent monoamine ] systems,<ref name="E Weihe" /> there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.<ref name="Burchett"/> |
|
'''Trace amines''' are an endogenous group of ]<ref name="pmid22038157">{{cite journal |vauthors=Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM | title = Trace amine associated receptor 1 signaling in activated lymphocytes | journal = J Neuroimmune Pharmacol | volume = 7 | issue = 4 | pages = 866–76 |date=December 2012 | pmid = 22038157 | pmc = 3593117 | doi = 10.1007/s11481-011-9321-4 | quote = Trace Amine Associated Receptor 1 (TAAR1) is a G protein coupled receptor (GPCR) that responds to a wide spectrum of agonists, including endogenous trace amines, ...}}</ref> – and hence, monoaminergic ]<ref name="Burchett">{{cite journal |vauthors=Burchett SA, Hicks TP | title = The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain | journal = Prog. Neurobiol. | volume = 79 | issue = 5–6 | pages = 223–46 |date=August 2006 | pmid = 16962229 | doi = 10.1016/j.pneurobio.2006.07.003 | s2cid = 10272684 }}</ref><ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> – that are structurally and metabolically related to classical ]s.<ref name="Vascular" /> Compared to the classical monoamines, they are present in trace concentrations.<ref name="Vascular" /> They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of ].<ref name="Vascular" /><ref name="Miller" /> Although they can be synthesized within parent monoamine ] systems,<ref name="E Weihe" /> there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.<ref name="Burchett"/> |
|
Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the ] of monoamine neurons with {{nowrap|co-localized}} {{abbr|TAAR1|trace amine-associated receptor 1}}.<ref name="Miller" /> They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via ] activation;<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> specifically, by activating TAAR1 in neurons they promote the release{{#tag:ref|Certain trace amines (e.g., ]) functionally inhibit the vesicular monoamine transporter ], while others do not (e.g., ]). The trace amines that do not inhibit ] function in monoamine neurons do not release neurotransmitters as effectively as those which do.|group="note"}} and prevent reuptake of monoamine neurotransmitters from the synaptic cleft as well as inhibit ].<ref name="Miller"/><ref name="Miller+Grandy 2016">{{cite journal | vauthors = Grandy DK, Miller GM, Li JX | title = "TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference | journal = Drug Alcohol Depend. | volume = 159 | pages = 9–16 | date = February 2016 | pmid = 26644139 | doi = 10.1016/j.drugalcdep.2015.11.014 | quote = TAAR1 is a high-affinity receptor for METH/AMPH and DA | pmc=4724540}}</ref> Phenethylamine and amphetamine possess analogous ] in human ]s, as both compounds induce efflux from ] (VMAT2)<ref name="E Weihe">{{cite journal |vauthors=Eiden LE, Weihe E | title = VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse | journal = Ann. N. Y. Acad. Sci. | volume = 1216 | pages = 86–98 |date=January 2011 | pmid = 21272013 | doi = 10.1111/j.1749-6632.2010.05906.x | quote= neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). | pmc=4183197}}</ref><ref name="Offermanns">{{cite book | editor1=Offermanns, S | editor2= Rosenthal, W| title=Encyclopedia of Molecular Pharmacology |year=2008|publisher=Springer|location=Berlin|isbn=978-3540389163|pages=1219–1222|edition=2nd}}</ref> and activate ] with comparable efficacy.<ref name="Miller" /> |
|
Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the ] of monoamine neurons with {{nowrap|co-localized}} {{abbr|TAAR1|trace amine-associated receptor 1}}.<ref name="Miller" /> They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via ] activation;<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> specifically, by activating TAAR1 in neurons they promote the release{{#tag:ref|Certain trace amines (e.g., ]) functionally inhibit the vesicular monoamine transporter ], while others do not (e.g., ]). The trace amines that do not inhibit ] function in monoamine neurons do not release neurotransmitters as effectively as those which do.|group="note"}} and prevent reuptake of monoamine neurotransmitters from the synaptic cleft as well as inhibit ].<ref name="Miller"/><ref name="Miller+Grandy 2016">{{cite journal | vauthors = Grandy DK, Miller GM, Li JX | title = "TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference | journal = Drug Alcohol Depend. | volume = 159 | pages = 9–16 | date = February 2016 | pmid = 26644139 | doi = 10.1016/j.drugalcdep.2015.11.014 | quote = TAAR1 is a high-affinity receptor for METH/AMPH and DA | pmc=4724540}}</ref> Phenethylamine and amphetamine possess analogous ] in human ]s, as both compounds induce efflux from ] (VMAT2)<ref name="E Weihe">{{cite journal |vauthors=Eiden LE, Weihe E | title = VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse | journal = Ann. N. Y. Acad. Sci. | volume = 1216 | pages = 86–98 |date=January 2011 | issue = 1 | pmid = 21272013 | doi = 10.1111/j.1749-6632.2010.05906.x | quote= neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). | pmc=4183197| bibcode = 2011NYASA1216...86E }}</ref><ref name="Offermanns">{{cite book | editor1=Offermanns, S | editor2= Rosenthal, W| title=Encyclopedia of Molecular Pharmacology |year=2008|publisher=Springer|location=Berlin|isbn=978-3540389163|pages=1219–1222|edition=2nd}}</ref> and activate ] with comparable efficacy.<ref name="Miller" /> |
|
Like ], ], and ], the trace amines have been implicated in a vast array of human disorders of affect and cognition, such as ],<ref name="Neuropsychopharm">{{cite journal | author = Berry MD | title = The potential of trace amines and their receptors for treating neurological and psychiatric diseases | journal = Rev Recent Clin Trials | volume = 2 | issue = 1 | pages = 3–19 |date=January 2007 | pmid = 18473983 | doi = 10.2174/157488707779318107| quote = changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD) . PE has been shown to induce hyperactivity and aggression, two of the cardinal clinical features of ADHD, in experimental animals . Hyperactivity is also a symptom of phenylketonuria, which as discussed above is associated with a markedly elevated PE turnover . Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors . Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients and has been reported to enhance the activity of PE at TAAR1 . Conversely, methylphenidate, which is also clinically useful in ADHD, showed poor efficacy at the TAAR1 receptor . In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1 .<br />More direct evidence has been obtained recently for a role of trace amines in ADHD. Urinary PE levels have been reported to be decreased in ADHD patients in comparison to both controls and patients with autism . Evidence for a decrease in PE levels in the brain of ADHD patients has also recently been reported . In addition, decreases in the urine and plasma levels of the PE metabolite phenylacetic acid and the precursors phenylalanine and tyrosine have been reported along with decreases in plasma tyramine . Following treatment with methylphenidate, patients who responded positively showed a normalization of urinary PE, whilst non-responders showed no change from baseline values .}}</ref><ref name="Renaissance GPCR" /><ref name="Review 2009" /> ]<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> and ],<ref name="Burchett"/><ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> among others.<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /><ref name="Review 2009">{{cite journal |vauthors=Sotnikova TD, Caron MG, Gainetdinov RR | title = Trace amine-associated receptors as emerging therapeutic targets | journal = Mol. Pharmacol. | volume = 76 | issue = 2 | pages = 229–35 |date=August 2009 | pmid = 19389919 | pmc = 2713119 | doi = 10.1124/mol.109.055970 | quote = Although the functional role of trace amines in mammals remains largely enigmatic, it has been noted that trace amine levels can be altered in various human disorders, including schizophrenia, Parkinson's disease, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, and phenylketonuria (Boulton, 1980; Sandler et al., 1980). It was generally held that trace amines affect the monoamine system indirectly via interaction with plasma membrane transporters and vesicular storage (Premont et al., 2001; Branchek and Blackburn, 2003; Berry, 2004; Sotnikova et al., 2004). ...<br />Furthermore, DAT-deficient mice provide a model to investigate the inhibitory actions of amphetamines on hyperactivity, the feature of amphetamines believed to be important for their therapeutic action in ADHD (Gainetdinov et al., 1999; Gainetdinov and Caron, 2003). It should be noted also that the best-established agonist of TAAR1, β-PEA, shared the ability of amphetamine to induce inhibition of dopamine-dependent hyperactivity of DAT-KO mice (Gainetdinov et al., 1999; Sotnikova et al., 2004).<br />Furthermore, if TAAR1 could be proven as a mediator of some of amphetamine's actions in vivo, the development of novel TAAR1-selective agonists and antagonists could provide a new approach for the treatment of amphetamine-related conditions such as addiction and/or disorders in which amphetamine is used therapeutically. In particular, because amphetamine has remained the most effective pharmacological treatment in ADHD for many years, a potential role of TAAR1 in the mechanism of the “paradoxical” effectiveness of amphetamine in this disorder should be explored.}}</ref> Trace aminergic hypo-function is particularly relevant to ], since urinary and ] phenethylamine concentrations are significantly lower in ADHD individuals relative to controls and the two most commonly prescribed drugs for ADHD, ] and ], increase phenethylamine biosynthesis in treatment-responsive individuals with ADHD.<ref name="Neuropsychopharm" /><ref name="Zinc and PEA">{{cite journal |vauthors=Scassellati C, Bonvicini C, Faraone SV, Gennarelli M | title = Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses | journal = J. Am. Acad. Child Adolesc. Psychiatry | volume = 51 | issue = 10 | pages = 1003–1019.e20 | date = October 2012 | pmid = 23021477 | doi = 10.1016/j.jaac.2012.08.015 | quote = Although we did not find a sufficient number of studies suitable for a meta-analysis of PEA and ADHD, three studies<sup>20,57,58</sup> confirmed that urinary levels of PEA were significantly lower in patients with ADHD compared with controls. ... Administration of D-amphetamine and methylphenidate resulted in a markedly increased urinary excretion of PEA,<sup>20,60</sup> suggesting that ADHD treatments normalize PEA levels. ... Similarly, urinary biogenic trace amine PEA levels could be a biomarker for the diagnosis of ADHD,<sup>20,57,58</sup> for treatment efficacy,<sup>20,60</sup> and associated with symptoms of inattentivenesss.<sup>59</sup> ... With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.<sup>110</sup>}}</ref> A ] of ADHD ]s also indicated that urinary phenethylamine levels could be a diagnostic biomarker for ADHD.<ref name="Zinc and PEA" /> |
|
Like ], ], and ], the trace amines have been implicated in a vast array of human disorders of affect and cognition, such as ],<ref name="Neuropsychopharm">{{cite journal | author = Berry MD | title = The potential of trace amines and their receptors for treating neurological and psychiatric diseases | journal = Rev Recent Clin Trials | volume = 2 | issue = 1 | pages = 3–19 |date=January 2007 | pmid = 18473983 | doi = 10.2174/157488707779318107| quote = changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD) . PE has been shown to induce hyperactivity and aggression, two of the cardinal clinical features of ADHD, in experimental animals . Hyperactivity is also a symptom of phenylketonuria, which as discussed above is associated with a markedly elevated PE turnover . Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors . Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients and has been reported to enhance the activity of PE at TAAR1 . Conversely, methylphenidate, which is also clinically useful in ADHD, showed poor efficacy at the TAAR1 receptor . In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1 .<br />More direct evidence has been obtained recently for a role of trace amines in ADHD. Urinary PE levels have been reported to be decreased in ADHD patients in comparison to both controls and patients with autism . Evidence for a decrease in PE levels in the brain of ADHD patients has also recently been reported . In addition, decreases in the urine and plasma levels of the PE metabolite phenylacetic acid and the precursors phenylalanine and tyrosine have been reported along with decreases in plasma tyramine . Following treatment with methylphenidate, patients who responded positively showed a normalization of urinary PE, whilst non-responders showed no change from baseline values .}}</ref><ref name="Renaissance GPCR" /><ref name="Review 2009" /> ]<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> and ],<ref name="Burchett"/><ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /> among others.<ref name="Neuropsychopharm" /><ref name="Renaissance GPCR" /><ref name="Review 2009">{{cite journal |vauthors=Sotnikova TD, Caron MG, Gainetdinov RR | title = Trace amine-associated receptors as emerging therapeutic targets | journal = Mol. Pharmacol. | volume = 76 | issue = 2 | pages = 229–35 |date=August 2009 | pmid = 19389919 | pmc = 2713119 | doi = 10.1124/mol.109.055970 | quote = Although the functional role of trace amines in mammals remains largely enigmatic, it has been noted that trace amine levels can be altered in various human disorders, including schizophrenia, Parkinson's disease, attention deficit hyperactivity disorder (ADHD), Tourette syndrome, and phenylketonuria (Boulton, 1980; Sandler et al., 1980). It was generally held that trace amines affect the monoamine system indirectly via interaction with plasma membrane transporters and vesicular storage (Premont et al., 2001; Branchek and Blackburn, 2003; Berry, 2004; Sotnikova et al., 2004). ...<br />Furthermore, DAT-deficient mice provide a model to investigate the inhibitory actions of amphetamines on hyperactivity, the feature of amphetamines believed to be important for their therapeutic action in ADHD (Gainetdinov et al., 1999; Gainetdinov and Caron, 2003). It should be noted also that the best-established agonist of TAAR1, β-PEA, shared the ability of amphetamine to induce inhibition of dopamine-dependent hyperactivity of DAT-KO mice (Gainetdinov et al., 1999; Sotnikova et al., 2004).<br />Furthermore, if TAAR1 could be proven as a mediator of some of amphetamine's actions in vivo, the development of novel TAAR1-selective agonists and antagonists could provide a new approach for the treatment of amphetamine-related conditions such as addiction and/or disorders in which amphetamine is used therapeutically. In particular, because amphetamine has remained the most effective pharmacological treatment in ADHD for many years, a potential role of TAAR1 in the mechanism of the “paradoxical” effectiveness of amphetamine in this disorder should be explored.}}</ref> Trace aminergic hypo-function is particularly relevant to ], since urinary and ] phenethylamine concentrations are significantly lower in ADHD individuals relative to controls and the two most commonly prescribed drugs for ADHD, ] and ], increase phenethylamine biosynthesis in treatment-responsive individuals with ADHD.<ref name="Neuropsychopharm" /><ref name="Zinc and PEA">{{cite journal |vauthors=Scassellati C, Bonvicini C, Faraone SV, Gennarelli M | title = Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses | journal = J. Am. Acad. Child Adolesc. Psychiatry | volume = 51 | issue = 10 | pages = 1003–1019.e20 | date = October 2012 | pmid = 23021477 | doi = 10.1016/j.jaac.2012.08.015 | quote = Although we did not find a sufficient number of studies suitable for a meta-analysis of PEA and ADHD, three studies<sup>20,57,58</sup> confirmed that urinary levels of PEA were significantly lower in patients with ADHD compared with controls. ... Administration of D-amphetamine and methylphenidate resulted in a markedly increased urinary excretion of PEA,<sup>20,60</sup> suggesting that ADHD treatments normalize PEA levels. ... Similarly, urinary biogenic trace amine PEA levels could be a biomarker for the diagnosis of ADHD,<sup>20,57,58</sup> for treatment efficacy,<sup>20,60</sup> and associated with symptoms of inattentivenesss.<sup>59</sup> ... With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.<sup>110</sup>}}</ref> A ] of ADHD ]s also indicated that urinary phenethylamine levels could be a diagnostic biomarker for ADHD.<ref name="Zinc and PEA" /> |
 Phenethylamine skeleton
Phenethylamine skeleton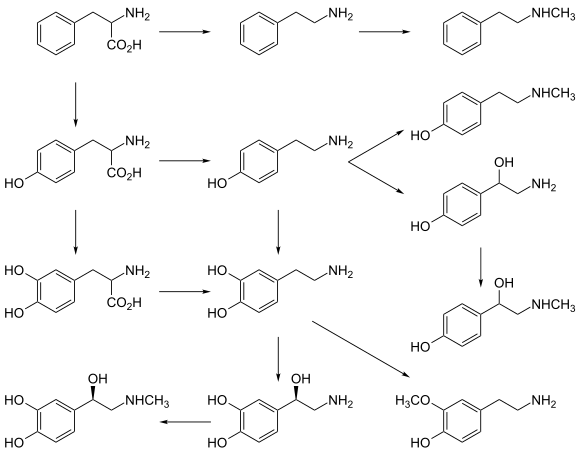 L-Phenylalanine
L-Tyrosine
L-DOPA
Epinephrine
Phenethylamine
p-Tyramine
Dopamine
Norepinephrine
N-Methylphenethylamine
N-Methyltyramine
p-Octopamine
Synephrine
3-Methoxytyramine
AADC
AADC
AADC
primary
L-Phenylalanine
L-Tyrosine
L-DOPA
Epinephrine
Phenethylamine
p-Tyramine
Dopamine
Norepinephrine
N-Methylphenethylamine
N-Methyltyramine
p-Octopamine
Synephrine
3-Methoxytyramine
AADC
AADC
AADC
primary