| Revision as of 07:56, 12 December 2021 editCitation bot (talk | contribs)Bots5,452,147 edits Add: bibcode, pmid, pages, authors 1-1. Removed parameters. Formatted dashes. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Abductive | #UCB_toolbar← Previous edit | Latest revision as of 21:45, 2 August 2023 edit undoInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,388,257 edits Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.9.5 | ||
| Line 57: | Line 57: | ||
| == External links == | == External links == | ||
| * | * {{Webarchive|url=https://web.archive.org/web/20100109131018/http://msds.chem.ox.ac.uk/AD/adipoyl_chloride.html |date=2010-01-09 }} | ||
| {{Navbox acyl chlorides}} | {{Navbox acyl chlorides}} | ||
Latest revision as of 21:45, 2 August 2023
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Hexanedioyl dichloride | |||
| Other names Adipoyl dichloride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 507709 | ||
| ChemSpider | |||
| ECHA InfoCard | 100.003.525 | ||
| EC Number |
| ||
| PubChem CID | |||
| UN number | 3265 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H8Cl2O2 | ||
| Molar mass | 183.03 g·mol | ||
| Density | 1.25 g/cm | ||
| Boiling point | 105 to 107 °C (221 to 225 °F; 378 to 380 K) at 2 mmHg | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 160 °C (320 °F; 433 K) (closed cup) | ||
| Related compounds | |||
| Related compounds | Adipic acid Hexanedihydrazide Hexanedinitrile Hexanediamide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
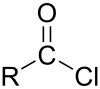
Adipoyl chloride (or adipoyl dichloride) is the organic compound with the formula (CH2CH2C(O)Cl)2. It is a colorless liquid. It reacts with water to give adipic acid.
It is prepared by treatment of adipic acid with thionyl chloride. Adipoyl chloride reacts with hexamethylenediamine to form nylon 6,6.
See also
References
- P. C. Guha; D. K. Sankaran (1946). "Muconic Acid". Organic Syntheses. 26: 57–60. doi:10.15227/orgsyn.026.0057. PMID 20280761.
- Morgan, Paul W.; Kwolek, Stephanie L. (April 1959). "The nylon rope trick: Demonstration of condensation polymerization". J. Chem. Educ. 36 (4): 182. Bibcode:1959JChEd..36..182M. doi:10.1021/ed036p182.
External links
- MSDS Safety data Archived 2010-01-09 at the Wayback Machine
| Diacyl chlorides (-COCl)2 | ||
|---|---|---|
|  | |
| Category:Acyl chlorides | ||

