| Revision as of 09:51, 14 April 2023 editDoroWolf (talk | contribs)392 editsNo edit summary← Previous edit | Latest revision as of 02:40, 3 August 2023 edit undoHeadbomb (talk | contribs)Edit filter managers, Autopatrolled, Extended confirmed users, Page movers, File movers, New page reviewers, Pending changes reviewers, Rollbackers, Template editors454,126 edits Alter: journal. | Use this tool. Report bugs. | #UCB_Gadget | ||
| Line 53: | Line 53: | ||
| }} | }} | ||
| '''Thymolphthalein''' is a ] used as an ]–] (]) ]. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The ] for the blue thymolphthalein ] is 38,000 M<sup>−1</sup> cm<sup>−1</sup> at 595 nm.<ref>{{cite journal |author=Hahn HH |author2=Cheuk SF |author3=Elfenbein S |author4= Wood WB |title= Studies on the Pathogenesis of Fever: Xix. Localization of Pyrogen in Granulocytes|journal= |
'''Thymolphthalein''' is a ] used as an ]–] (]) ]. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The ] for the blue thymolphthalein ] is 38,000 M<sup>−1</sup> cm<sup>−1</sup> at 595 nm.<ref>{{cite journal |author=Hahn HH |author2=Cheuk SF |author3=Elfenbein S |author4= Wood WB |title= Studies on the Pathogenesis of Fever: Xix. Localization of Pyrogen in Granulocytes|journal= The Journal of Experimental Medicine|volume=131 |issue=4 |pages=701–9 |date=April 1970 |pmid=5430784 |pmc=2138774 |doi= 10.1084/jem.131.4.701}}</ref> | ||
| {{pH_indicator_template|indicator_name=Thymolphthalein |low_pH=9.3 |high_pH=10.5|low_pH_color=white|high_pH_color=blue|high_pH_text=white}} | {{pH_indicator_template|indicator_name=Thymolphthalein |low_pH=9.3 |high_pH=10.5|low_pH_color=white|high_pH_color=blue|high_pH_text=white}} | ||
| Thymolphthalein is also known to have use as a ]<ref>{{cite journal | last1 = Hubacher | first1 = MH | last2 = Doernberg | first2 = S | last3 = Horner | first3 = A | year = 1953 | title = Laxatives: chemical structure and potency of phthaleins and hydroxyanthraquinones | journal = |
Thymolphthalein is also known to have use as a ]<ref>{{cite journal | last1 = Hubacher | first1 = MH | last2 = Doernberg | first2 = S | last3 = Horner | first3 = A | year = 1953 | title = Laxatives: chemical structure and potency of phthaleins and hydroxyanthraquinones | journal = Journal of the American Pharmaceutical Association | volume = 42 | issue = 1| pages = 23–30 | pmid = 13034620 | doi = 10.1002/jps.3030420108 }}</ref> and for ].<ref>{{Cite web|url=https://www.chymist.com/Disappearing%20Ink.pdf|title=Disappearing Ink|last=Katz|first=David A.|date=1982|website=www.chymist.com|access-date=August 14, 2017}}</ref> | ||
| ==Preparation== | ==Preparation== | ||
Latest revision as of 02:40, 3 August 2023
This article is about thymolphthalein. For other related dyes in the phthalein family, see Phthalein dye.
| |
| Names | |
|---|---|
| Preferred IUPAC name 3,3-Bis-2-benzofuran-1(3H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.300 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H30O4 |
| Molar mass | 430.544 g·mol |
| Appearance | White powder |
| Melting point | 248 to 252 °C (478 to 486 °F; 521 to 525 K) (decomposes) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H341, H350, H361 |
| Precautionary statements | P201, P202, P210, P233, P240, P241, P242, P243, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thymolphthalein is a phthalein dye used as an acid–base (pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is 38,000 M cm at 595 nm.
| Thymolphthalein (pH indicator) | ||
| below pH 9.3 | above pH 10.5 | |
| 9.3 | ⇌ | 10.5 |
Thymolphthalein is also known to have use as a laxative and for disappearing ink.
Preparation
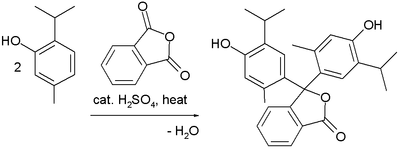
Thymolphthalein can be synthesized from thymol and phthalic anhydride.
See also
References
- "Thymolphthalein". pubchem.ncbi.nlm.nih.gov.
- Hahn HH; Cheuk SF; Elfenbein S; Wood WB (April 1970). "Studies on the Pathogenesis of Fever: Xix. Localization of Pyrogen in Granulocytes". The Journal of Experimental Medicine. 131 (4): 701–9. doi:10.1084/jem.131.4.701. PMC 2138774. PMID 5430784.
- Hubacher, MH; Doernberg, S; Horner, A (1953). "Laxatives: chemical structure and potency of phthaleins and hydroxyanthraquinones". Journal of the American Pharmaceutical Association. 42 (1): 23–30. doi:10.1002/jps.3030420108. PMID 13034620.
- Katz, David A. (1982). "Disappearing Ink" (PDF). www.chymist.com. Retrieved August 14, 2017.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |