| Revision as of 17:06, 20 November 2021 editDavemck (talk | contribs)Extended confirmed users119,673 editsm rmv duplicate parm← Previous edit | Revision as of 14:41, 30 September 2023 edit undoTrappist the monk (talk | contribs)Administrators479,784 editsm cite repair;Tag: AWBNext edit → | ||
| Line 11: | Line 11: | ||
| | ImageAlt1 = Ball-and-stick model of the thioacetamide molecule | | ImageAlt1 = Ball-and-stick model of the thioacetamide molecule | ||
| | IUPACName = Thioacetamide | | IUPACName = Thioacetamide | ||
| | PIN = Ethanethioamide<ref>{{cite book |author=] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=] | |
| PIN = Ethanethioamide<ref>{{cite book |author=] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=] |page=856 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> | ||
| | OtherNames = acetothioamide, TAA, thioacetimidic acid, TA, TAM | | OtherNames = acetothioamide, TAA, thioacetimidic acid, TA, TAM | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| Line 86: | Line 86: | ||
| ==Structure== | ==Structure== | ||
| The C<sub>2</sub>NH<sub>2</sub>S portion of the molecule is planar; the C-S, C-N, and C-C distances are 1.68, 1.31, and 1.50 Å, respectively. The short C-S and C-N distances indicate multiple bonding.<ref name=Hurst>{{ cite journal | |
The C<sub>2</sub>NH<sub>2</sub>S portion of the molecule is planar; the C-S, C-N, and C-C distances are 1.68, 1.31, and 1.50 Å, respectively. The short C-S and C-N distances indicate multiple bonding.<ref name=Hurst>{{ cite journal |author=Trevor W. Hambley |author2=David E. Hibbs |author3=Peter Turner |author4=Siân. T. Howard |author5=Michael B. Hursthouse | title = Insights into Bonding and Hydrogen Bond Directionality in Thioacetamide from the Experimental Charge Distribution | journal = J. Chem. Soc., Perkin Trans. | year = 2002| pages = 235–239| doi = 10.1039/B109353C | issue=2}}</ref> | ||
| ==Safety== | ==Safety== | ||
| Line 95: | Line 95: | ||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| * {{cite web | title = Thioacetamide (Sulfo amine) | work = Chemical Land 21 | url = http://www.chemicalland21.com/specialtychem/finechem/THIOACETAMIDE.htm | |
* {{cite web | title = Thioacetamide (Sulfo amine) | work = Chemical Land 21 | url = http://www.chemicalland21.com/specialtychem/finechem/THIOACETAMIDE.htm | access-date = February 14, 2006 }} | ||
| {{Authority control}} | {{Authority control}} | ||
Revision as of 14:41, 30 September 2023
| |
| |
| Names | |
|---|---|
| IUPAC name Thioacetamide | |
| Preferred IUPAC name Ethanethioamide | |
| Other names acetothioamide, TAA, thioacetimidic acid, TA, TAM | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 506006 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.493 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H5NS |
| Molar mass | 75.13 g/mol |
| Appearance | colourless crystals |
| Odor | slight mercaptan |
| Density | 1.319 g/cm |
| Melting point | 115 °C (239 °F; 388 K) |
| Boiling point | decomposes |
| Solubility in water | good |
| Magnetic susceptibility (χ) | -42.45·10 cm/mol |
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Foul stench, carcinogenic |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H350, H412 |
| Precautionary statements | P201, P202, P264, P270, P273, P280, P281, P301+P312, P302+P352, P305+P351+P338, P308+P313, P321, P330, P332+P313, P337+P313, P362, P405, P501 |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
| Related compounds | acetamide, dithioacetic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
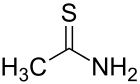
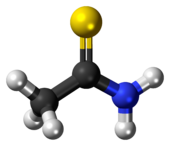
Thioacetamide is an organosulfur compound with the formula C2H5NS. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide.
Research
Thioacetamide is known to induce acute or chronic liver disease (fibrosis and cirrhosis) in the experimental animal model. Its administration in rat induces hepatic encephalopathy, metabolic acidosis, increased levels of transaminases, abnormal coagulopathy, and centrilobular necrosis, which are the main features of the clinical chronic liver disease so thioacetamide can precisely replicate the initiation and progression of human liver disease in an experimental animal model.
Coordination chemistry
Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions. Thus, treatment of aqueous solutions of many metal cations to a solution of thioacetamide affords the corresponding metal sulfide:
- M + CH3C(S)NH2 + H2O → MS + CH3C(O)NH2 + 2 H (M = Ni, Pb, Cd, Hg)
Related precipitations occur for sources of soft trivalent cations (As, Sb, Bi) and monovalent cations (Ag, Cu).
Preparation
Thioacetamide is prepared by treating acetamide with phosphorus pentasulfide as shown in the following idealized reaction:
- CH3C(O)NH2 + 1/4 P4S10 → CH3C(S)NH2 + 1/4 P4S6O4
Structure
The C2NH2S portion of the molecule is planar; the C-S, C-N, and C-C distances are 1.68, 1.31, and 1.50 Å, respectively. The short C-S and C-N distances indicate multiple bonding.
Safety
Thioacetamide is carcinogen class 2B.
It is known to produce marked hepatotoxicity in exposed animals. Toxicity values are 301 mg/kg in rats (LD50, oral administration), 300 mg/kg in mice (LD50, intraperitoneal administration). This is evidenced by enzymatic changes, which include elevation in the levels of serum alanine transaminase, aspartate transaminase and aspartic acid.
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 856. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Trevor W. Hambley; David E. Hibbs; Peter Turner; Siân. T. Howard; Michael B. Hursthouse (2002). "Insights into Bonding and Hydrogen Bond Directionality in Thioacetamide from the Experimental Charge Distribution". J. Chem. Soc., Perkin Trans. (2): 235–239. doi:10.1039/B109353C.
- Dwivedi DK, Jena GB (2018). "Glibenclamide protects against thioacetamide-induced hepatic damage in Wistar rat: investigation on NLRP3, MMP-2, and stellate cell activation". Naunyn-Schmiedeberg's Archives of Pharmacology. 391 (11): 1257–1274. doi:10.1007/s00210-018-1540-2. PMID 30066023. S2CID 51890984.
- Schwarz, G. (1945). "2,4-Dimethylthiazole". Organic Syntheses. 25: 35; Collected Volumes, vol. 3, p. 332.
- "HSDB: THIOACETAMIDE CASRN: 62-55-5". Hazardous Substances Data Bank.
- Ali, S.; Ansari, K. A.; Jafry, M. A.; Kabeer, H.; Diwakar, G. (2000). "Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats". Journal of Ethnopharmacology. 71 (3): 359–363. doi:10.1016/S0378-8741(99)00153-1. PMID 10940571.
- "Thioacetamide (Sulfo amine)". Chemical Land 21. Retrieved February 14, 2006.