| Revision as of 16:32, 29 April 2023 editLegionMammal978 (talk | contribs)Extended confirmed users7,894 edits move systematic name← Previous edit | Latest revision as of 00:08, 9 November 2023 edit undoOAbot (talk | contribs)Bots440,440 editsm Open access bot: doi updated in citation with #oabot. | ||
| Line 43: | Line 43: | ||
| '''Ayanin''' is an ], a type of flavonoid. It is the 3,7,4'-tri-''O''-methylated ] of ]. | '''Ayanin''' is an ], a type of flavonoid. It is the 3,7,4'-tri-''O''-methylated ] of ]. | ||
| It can be found in '']''. It can also be synthesized.<ref>{{cite journal | doi = 10.1002/jhet.5570130629| title = Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin| journal = Journal of Heterocyclic Chemistry| volume = 13| issue = 6| pages = 1293–1295| year = 1976| last1 = Rao| first1 = Koppaka V.| last2 = Owoyale| first2 = Jacob A.}}</ref> | It can be found in '']''. It can also be synthesized.<ref>{{cite journal | doi = 10.1002/jhet.5570130629| title = Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin| journal = Journal of Heterocyclic Chemistry| volume = 13| issue = 6| pages = 1293–1295| year = 1976| last1 = Rao| first1 = Koppaka V.| last2 = Owoyale| first2 = Jacob A.| doi-access = free}}</ref> | ||
| == Biosynthesis == | == Biosynthesis == | ||
Latest revision as of 00:08, 9 November 2023
| |
| Names | |
|---|---|
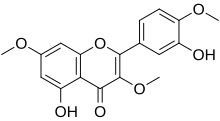
| IUPAC name 3′,5-Dihydroxy-3,4′,7-trimethoxyflavone | |
| Systematic IUPAC name 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,7-dimethoxy-4H-1-benzopyran-4-one | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H16O7 |
| Molar mass | 344.319 g·mol |
| Density | 1.454 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ayanin is an O-methylated flavonol, a type of flavonoid. It is the 3,7,4'-tri-O-methylated derivative of quercetin.
It can be found in Croton schiedeanus. It can also be synthesized.
Biosynthesis
The enzyme 3,7-dimethylquercetin 4'-O-methyltransferase uses S-adenosyl methionine and rhamnazin to produce S-adenosylhomocysteine and ayanin.
References
- Rao, Koppaka V.; Owoyale, Jacob A. (1976). "Partial methylation of quercetin: Direct synthesis of tamarixetin, ombuin and ayanin". Journal of Heterocyclic Chemistry. 13 (6): 1293–1295. doi:10.1002/jhet.5570130629.
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |