| Revision as of 10:56, 2 March 2023 editInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,380,770 edits Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.9.3) (Whoop whoop pull up - 12538← Previous edit | Revision as of 14:23, 2 December 2023 edit undoThumperward (talk | contribs)Administrators122,782 edits lots of general cleanupNext edit → | ||
| Line 1: | Line 1: | ||
| {{Short description|Acetylene-burning lamps}} | {{Short description|Acetylene-burning lamps}} | ||
| {{See also|Headlamp (outdoor)}} | |||
| {{more citations needed|date=July 2015}} | {{more citations needed|date=July 2015}} | ||
| ] | ] | ||
| '''Carbide lamps''', or '''acetylene gas lamps''', are simple lamps that produce and burn ] (C<sub>2</sub>H<sub>2</sub>) which is created by the reaction of ] (CaC<sub>2</sub>) with ] (H<sub>2</sub>O).<ref name=handbook>{{Cite book| last =Patnaik | first =Pradyot | year = 2003 | title =Handbook of Inorganic Chemical Compounds | publisher = McGraw-Hill | isbn =0-07-049439-8 | url= https://books.google.com/books?id=Xqj-TTzkvTEC&pg=PA243 }}</ref> | |||
| A '''Carbide lamp''' or '''acetylene gas lamp''' is a simple lamp that produces and burns ] (C<sub>2</sub>H<sub>2</sub>), which is created by the reaction of ] (CaC<sub>2</sub>) with ] (H<sub>2</sub>O).<ref name=handbook>{{Cite book| last =Patnaik | first =Pradyot | year = 2003 | title =Handbook of Inorganic Chemical Compounds | publisher = McGraw-Hill | isbn =0-07-049439-8 | url= https://books.google.com/books?id=Xqj-TTzkvTEC&pg=PA243 }}</ref> | |||
| Acetylene gas lamps were used to illuminate buildings, as ] beacons, and as headlights on motor-cars and bicycles. Portable acetylene gas lamps, worn on the hat or carried by hand, were widely used in mining in the early twentieth century. They are still employed by ]s, hunters, and ]s. Small carbide lamps called "carbide candles" or "smokers" are used for blackening rifle sights to reduce glare. They are sometimes referred to as "smokers" because of the ]y flame produced by acetylene.<ref name="smoker">{{cite video|url=https://www.youtube.com/watch?t=4&v=rXF0u8OrLdU|title=Using the Super Smoker|publisher=ray-vin.com|access-date=11 October 2015}}</ref> | |||
| Acetylene gas lamps were used to illuminate buildings, as ] beacons, and as headlights on motor-cars and bicycles. Portable acetylene gas lamps, worn on the hat or carried by hand, were widely used in mining in the early twentieth century. They are still employed by ]s, hunters, and ]s. | |||
| ==Mechanism== | |||
| == History == | |||
| ] | |||
| ]]] | |||
| In 1892, ] discovered an economically efficient process for creating calcium carbide in an ] from a mixture of lime and coke. The arc furnace provides the high temperature required to drive the reaction.<ref>{{Cite journal | title = The Manufacture of Calcium Carbide | author = Morehead, J. T. and de Chalmot, G. | journal = Journal of the American Chemical Society | pages = 311–331 | doi = 10.1021/ja02090a001 | volume = 18 | issue = 4 | year = 1896| url = https://zenodo.org/record/1428938 }}</ref> Manufacture of calcium carbide was an important part of the industrial revolution in chemistry, and was made possible in the United States as a result of massive amounts of inexpensive hydroelectric power produced at ] before the turn of the twentieth century.<ref>{{cite journal|last=Freeman|first=Horace|title=Manufacture of Cyanamide|journal=The Chemical News and the Journal of Physical Science|year=1919|volume=117|page=232|url=https://books.google.com/books?id=5SAzAQAAMAAJ&pg=PA232}}</ref> In 1895, Willson sold his patent to ]. Domestic lighting with acetylene gas was introduced circa 1894 and bicycle lamps from 1896. In France, ], a Parisian electrical engineer, also made domestic acetylene lamps and ]. | |||
| The first carbide bicycle lamp developed in the United States was ]ed in ] on August 28, 1900 by Frederick Baldwin.<ref>{{US patent|656874}}</ref> Another early lamp design is shown in a ] from ] from October 21, 1902.<ref>{{US patent|711871}}</ref> In the early 1900s, ] invented the ]. This combined two of Dalén's previous inventions: the substrate ] and the ]. Inventions and improvements to carbide lamps continued for decades.<ref>For example on March 10, 1925 Andrew Prader of ] was granted {{US Patent|1,528,848}}</ref> | |||
| A mining or caving lamp has ] placed in a lower chamber, the generator. The upper reservoir is then filled with water. A threaded valve or other mechanism is used to control the rate at which the water is allowed to drip into the chamber containing the calcium carbide. By controlling the rate of water flow, the production of ] gas is controlled. This, in turn, controls the flow rate of the gas and the size of the flame at the burner, and thus the amount of light it produces. | |||
| After carbide lamp open flames were implicated in an Illinois coal-seam methane gas explosion that killed 54 miners, the ],<ref>Fifty-First Annual Coal Report of Illinois, 1932, Department of Mines and Minerals. Journal Printing Co.: Springfield, ILL., 1933</ref> carbide lamp use declined in United States coal mines. They continued to be used in the coal pits of other countries, notably ]. | |||
| This type of lamp generally has a reflector behind the flame to help project the light forward. An acetylene gas powered lamp produces a bright, broad light. Many cavers prefer this type of unfocused light as it improves peripheral vision in the complete dark. The reaction of carbide with water is exothermic and produces a fair amount of heat independent of the flame. In cold caves, carbide lamp users can use this heat to help stave off hypothermia.<ref>Matthews, C. E. (1996). An illuminating reaction. The Science Teacher, 63(5), 30.</ref> | |||
| In the birth of the ], a carbide lamp was used as light support to ] the first film in the ], in 1900. | |||
| == Mechanism == | |||
| ] | |||
| {{See also|Calcium carbide#Production of acetylene}} | |||
| A mining or caving lamp has ] placed in a lower chamber, the generator. The upper reservoir is then filled with water. A threaded valve or other mechanism is used to control the rate at which the water is allowed to drip into the chamber containing the calcium carbide. By controlling the rate of water flow, the production of ] gas is controlled. This, in turn, controls the flow rate of the gas and the size of the flame at the burner, and thus the amount of light it produces. | |||
| This type of lamp generally has a reflector behind the flame to help project the light forward. An acetylene gas powered lamp produces a bright, broad light. Many cavers prefer this type of unfocused light as it improves peripheral vision in the complete dark. The reaction of carbide with water is exothermic and produces a fair amount of heat independent of the flame. In cold caves, carbide lamp users can use this heat to help stave off hypothermia.<ref>Matthews, C. E. (1996). An illuminating reaction. The Science Teacher, 63(5), 30.</ref> | |||
| === Chemical reactions === | |||
| {{See also|Calcium carbide#Production of acetylene|label 1=Production of acetylene}} | |||
| Acetylene is produced by the reaction:<ref>{{Cite news|url=https://melscience.com/en/articles/chemical-characteristics-calcium-carbide-and-its-r/|title=Chemical characteristics of calcium carbide and its reaction with water|work=MEL Science|access-date=2018-05-16|language=en}}</ref> | Acetylene is produced by the reaction:<ref>{{Cite news|url=https://melscience.com/en/articles/chemical-characteristics-calcium-carbide-and-its-r/|title=Chemical characteristics of calcium carbide and its reaction with water|work=MEL Science|access-date=2018-05-16|language=en}}</ref> | ||
| Line 28: | Line 36: | ||
| When all of the carbide in a lamp has been reacted, the carbide chamber contains a wet paste of slaked lime (]) which can be used to make a ]. This is emptied into a waste bag and the chamber can be refilled. | When all of the carbide in a lamp has been reacted, the carbide chamber contains a wet paste of slaked lime (]) which can be used to make a ]. This is emptied into a waste bag and the chamber can be refilled. | ||
| == |
== Uses == | ||
| ]]] | |||
| In 1892, ] discovered an economically efficient process for creating calcium carbide in an ] from a mixture of lime and coke. The arc furnace provides the high temperature required to drive the reaction.<ref>{{Cite journal | |||
| | title = The Manufacture of Calcium Carbide | |||
| | author = Morehead, J. T. and de Chalmot, G. | |||
| | journal = Journal of the American Chemical Society | |||
| | pages = 311–331 | |||
| | doi = 10.1021/ja02090a001 | |||
| | volume = 18 | |||
| | issue = 4 | |||
| | year = 1896| url = https://zenodo.org/record/1428938 | |||
| }}</ref> Manufacture of calcium carbide was an important part of the industrial revolution in chemistry, and was made possible in the United States as a result of massive amounts of inexpensive hydroelectric power produced at ] before the turn of the twentieth century.<ref>{{cite journal|last=Freeman|first=Horace|title=Manufacture of Cyanamide|journal=The Chemical News and the Journal of Physical Science|year=1919|volume=117|page=232|url=https://books.google.com/books?id=5SAzAQAAMAAJ&pg=PA232}}</ref> In 1895, Willson sold his patent to ]. Domestic lighting with acetylene gas was introduced circa 1894 and bicycle lamps from 1896. In France, ], a Parisian electrical engineer, also made domestic acetylene lamps and ]. | |||
| === Lighting systems === | |||
| The first carbide bicycle lamp developed in the United States was ]ed in ] on August 28, 1900 by Frederick Baldwin.<ref>{{US patent|656874}}</ref> Another early lamp design is shown in a ] from ] from October 21, 1902.<ref>{{US patent|711871}}</ref> In the early 1900s, ] invented the ]. This combined two of Dalén's previous inventions: the substrate ] and the ]. Inventions and improvements to carbide lamps continued for decades.<ref>For example on March 10, 1925 Andrew Prader of ] was granted {{US Patent|1,528,848}}</ref> | |||
| ] | |||
| After carbide lamp open flames were implicated in an Illinois coal-seam methane gas explosion that killed 54 miners, the ],<ref>Fifty-First Annual Coal Report of Illinois, 1932, Department of Mines and Minerals. Journal Printing Co.: Springfield, ILL., 1933</ref> carbide lamp use declined in United States coal mines. They continued to be used in the coal pits of other countries, notably ]. | |||
| In the birth of the ], a carbide lamp was used as light support to ] the first film in the ], in 1900. | |||
| ==Lighting systems == | |||
| ] | |||
| Carbide lighting was used in rural and urban areas of the United States which were not served by ]. Its use began shortly after 1900 and continued past 1950. ] pellets were placed in a container outside the home, with water piped to the container and allowed to drip on the pellets releasing acetylene. This gas was piped to ]s inside the house, where it was burned, creating a very bright flame. Carbide lighting was inexpensive but was prone to ]s and explosions. | Carbide lighting was used in rural and urban areas of the United States which were not served by ]. Its use began shortly after 1900 and continued past 1950. ] pellets were placed in a container outside the home, with water piped to the container and allowed to drip on the pellets releasing acetylene. This gas was piped to ]s inside the house, where it was burned, creating a very bright flame. Carbide lighting was inexpensive but was prone to ]s and explosions. | ||
| Early models of the automobile, car, motorbike and bicycle used carbide lamps as ]s. |
Early models of the automobile, car, motorbike and bicycle used carbide lamps as ]s. Acetylene gas, derived from carbide, enabled early automobiles to drive safely at night. Thick concave mirrors combined with magnifying lenses projected the acetylene flame light. These type of lights were used until reliable batteries and dynamos became available, and manufacturers switched to electric lights. | ||
| Acetylene lamps were also used on riverboats for night navigation. The ] has a lamp made in about 1910 that was used on board {{PS|Enterprise}}, a ] which has been restored to working order and also in the museum's collection.<ref>{{Cite web |url=http://www.nma.gov.au/collections/highlights/paddle-steamer-enterprise |title=Paddle Steamer Enterprise, National Museum of Australia |access-date=2012-01-30 |archive-date=2018-09-23 |archive-url=https://web.archive.org/web/20180923111619/http://www.nma.gov.au/collections/highlights/paddle-steamer-enterprise |url-status=dead }}</ref> | Acetylene lamps were also used on riverboats for night navigation. The ] has a lamp made in about 1910 that was used on board {{PS|Enterprise}}, a ] which has been restored to working order and also in the museum's collection.<ref>{{Cite web |url=http://www.nma.gov.au/collections/highlights/paddle-steamer-enterprise |title=Paddle Steamer Enterprise, National Museum of Australia |access-date=2012-01-30 |archive-date=2018-09-23 |archive-url=https://web.archive.org/web/20180923111619/http://www.nma.gov.au/collections/highlights/paddle-steamer-enterprise |url-status=dead }}</ref> | ||
| Line 58: | Line 49: | ||
| They are also used for ]. | They are also used for ]. | ||
| == |
=== Caving === | ||
| {{Original research|section|date=February 2022}} | {{Original research|section|date=February 2022}} | ||
| ] | ] | ||
| ], Norway]] | ], Norway]] | ||
| ] excursions |
] excursions]] | ||
| Early ] enthusiasts, not yet having the advantage of light-weight electrical illumination, introduced the carbide lamp to their hobby.<ref> (from ])</ref> While increasingly replaced by more modern choices, a substantial percentage of cavers still use this method{{Citation needed|date=June 2021}}. | Early ] enthusiasts, not yet having the advantage of light-weight electrical illumination, introduced the carbide lamp to their hobby.<ref> (from ])</ref> While increasingly replaced by more modern choices, a substantial percentage of cavers still use this method{{Citation needed|date=June 2021}}. | ||
| Many cavers favour carbide lamps for their durability and quality of illumination. They were once favoured for their relative illumination per mass of fuel compared to battery powered devices{{Citation needed|date=January 2022}}. Before the advent of high-intensity ] illumination with lithium ion batteries, carbide also had two important advantages over the alternative of miners electric lamps. Miners lamps were intended to last for the duration of a standard working shift, whilst major caving explorations could be much longer, so the carbide could be replenished during the trip. Expeditions involving camping over several days in remote regions might not have access to electricity for recharging. Lamps used in such circumstances would consist of a belt mounted gas generator linked by flexible pipe to a headset{{Citation needed|date=January 2022}}. | Many cavers favour carbide lamps for their durability and quality of illumination. They were once favoured for their relative illumination per mass of fuel compared to battery powered devices{{Citation needed|date=January 2022}}. Before the advent of high-intensity ] illumination with lithium ion batteries, carbide also had two important advantages over the alternative of miners electric lamps. Miners lamps were intended to last for the duration of a standard working shift, whilst major caving explorations could be much longer, so the carbide could be replenished during the trip. Expeditions involving camping over several days in remote regions might not have access to electricity for recharging. Lamps used in such circumstances would consist of a belt mounted gas generator linked by flexible pipe to a headset{{Citation needed|date=January 2022}}. | ||
| Line 70: | Line 62: | ||
| The lamps are sometimes called "stinkies" because of their odour.<ref>{{Cite web|url=https://www-sop.inria.fr/agos-sophia/sis/slang.html|title=Caver's Slang|website=www-sop.inria.fr}}</ref> | The lamps are sometimes called "stinkies" because of their odour.<ref>{{Cite web|url=https://www-sop.inria.fr/agos-sophia/sis/slang.html|title=Caver's Slang|website=www-sop.inria.fr}}</ref> | ||
| === Glare reduction === | |||
| ==See also== | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| Small carbide lamps called "carbide candles" or "smokers" are used for blackening rifle sights to reduce glare. They are sometimes referred to as "smokers" because of the ]y flame produced by acetylene.<ref name="smoker">{{cite video|url=https://www.youtube.com/watch?t=4&v=rXF0u8OrLdU|title=Using the Super Smoker|publisher=ray-vin.com|access-date=11 October 2015}}</ref> | |||
| ==Notes== | |||
| == See also == | |||
| * {{anl|Dalén light}} | |||
| * {{anl|Flashlight}} | |||
| * {{anl|Trouble light}} | |||
| == Notes == | |||
| {{Reflist}} | {{Reflist}} | ||
| ==References== | == References == | ||
| * {{cite book | |||
| * {{cite book | last = Clemmer | first = Gregg | title = American Miners' Carbide Lamps: A Collectors Guide to American Carbide Mine Lighting | publisher = Westernlore Publications | year = 1987 | isbn = 978-0870260643 }} | |||
| | last = Clemmer | |||
| * {{cite book | last = Pohs | first = Henry | title = The Miners Flame Light Book | publisher = Flame Publishing | year = 1995 | isbn = 978-0964116504 }} | |||
| | first = Gregg | |||
| * {{cite book | last = Card | first = Peter W. | title = Early Vehicle Lighting | publisher = ] | date=October 2004 | isbn = 978-0-7478-0585-4 }} | |||
| | title = American Miners' Carbide Lamps: A Collectors Guide to American Carbide Mine Lighting | |||
| * {{cite book | last = Thorpe | first = Dave | title = Carbide Light: The Last Flame in American Mines | publisher = Bergamot Publishing | year = 2005 | isbn = 978-0976090526 }} | |||
| | publisher = Westernlore Publications | |||
| | year = 1987 | |||
| == External links == | |||
| | isbn = 978-0870260643 | |||
| {{commonscat|Carbide lamps}} | |||
| }} | |||
| * {{cite book | |||
| | last = Pohs | |||
| | first = Henry | |||
| | title = The Miners Flame Light Book | |||
| | publisher = Flame Publishing | |||
| | year = 1995 | |||
| | isbn = 978-0964116504 | |||
| }} | |||
| * {{cite book | |||
| | last = Card | |||
| | first = Peter W. | |||
| | title = Early Vehicle Lighting | |||
| | publisher = ] | |||
| | date=October 2004 | |||
| | isbn = 978-0-7478-0585-4 | |||
| }} | |||
| * {{cite book | |||
| | last = Thorpe | |||
| | first = Dave | |||
| | title = Carbide Light: The Last Flame in American Mines | |||
| | publisher = Bergamot Publishing | |||
| | year = 2005 | |||
| | isbn = 978-0976090526 | |||
| }} | |||
| ==External links== | |||
| {{Commons category|Carbide lamps}} | |||
| * A comprehensive guide to the care and maintenance of acetylene gas lamps | * A comprehensive guide to the care and maintenance of acetylene gas lamps | ||
| * Has many good pictures & videos. | * Has many good pictures & videos. | ||
Revision as of 14:23, 2 December 2023
Acetylene-burning lamps| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Carbide lamp" – news · newspapers · books · scholar · JSTOR (July 2015) (Learn how and when to remove this message) |

A Carbide lamp or acetylene gas lamp is a simple lamp that produces and burns acetylene (C2H2), which is created by the reaction of calcium carbide (CaC2) with water (H2O).
Acetylene gas lamps were used to illuminate buildings, as lighthouse beacons, and as headlights on motor-cars and bicycles. Portable acetylene gas lamps, worn on the hat or carried by hand, were widely used in mining in the early twentieth century. They are still employed by cavers, hunters, and cataphiles.
History

In 1892, Thomas Willson discovered an economically efficient process for creating calcium carbide in an electric arc furnace from a mixture of lime and coke. The arc furnace provides the high temperature required to drive the reaction. Manufacture of calcium carbide was an important part of the industrial revolution in chemistry, and was made possible in the United States as a result of massive amounts of inexpensive hydroelectric power produced at Niagara Falls before the turn of the twentieth century. In 1895, Willson sold his patent to Union Carbide. Domestic lighting with acetylene gas was introduced circa 1894 and bicycle lamps from 1896. In France, Gustave Trouvé, a Parisian electrical engineer, also made domestic acetylene lamps and gasometers.
The first carbide bicycle lamp developed in the United States was patented in New York on August 28, 1900 by Frederick Baldwin. Another early lamp design is shown in a patent from Duluth, Minnesota from October 21, 1902. In the early 1900s, Gustaf Dalén invented the Dalén light. This combined two of Dalén's previous inventions: the substrate Agamassan and the Sun valve. Inventions and improvements to carbide lamps continued for decades.
After carbide lamp open flames were implicated in an Illinois coal-seam methane gas explosion that killed 54 miners, the 1932 Moweaqua Coal Mine disaster, carbide lamp use declined in United States coal mines. They continued to be used in the coal pits of other countries, notably USSR.
In the birth of the cinema of Iquitos, a carbide lamp was used as light support to project the first film in the Casa de Fierro, in 1900.
Mechanism
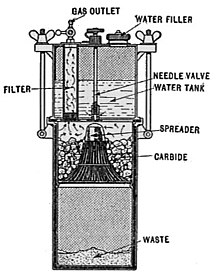
A mining or caving lamp has calcium carbide placed in a lower chamber, the generator. The upper reservoir is then filled with water. A threaded valve or other mechanism is used to control the rate at which the water is allowed to drip into the chamber containing the calcium carbide. By controlling the rate of water flow, the production of acetylene gas is controlled. This, in turn, controls the flow rate of the gas and the size of the flame at the burner, and thus the amount of light it produces.
This type of lamp generally has a reflector behind the flame to help project the light forward. An acetylene gas powered lamp produces a bright, broad light. Many cavers prefer this type of unfocused light as it improves peripheral vision in the complete dark. The reaction of carbide with water is exothermic and produces a fair amount of heat independent of the flame. In cold caves, carbide lamp users can use this heat to help stave off hypothermia.
Acetylene is produced by the reaction:
The acetylene combusts easily in the atmosphere:
When all of the carbide in a lamp has been reacted, the carbide chamber contains a wet paste of slaked lime (Ca(OH)2) which can be used to make a cement. This is emptied into a waste bag and the chamber can be refilled.
Uses
Lighting systems

Carbide lighting was used in rural and urban areas of the United States which were not served by electrification. Its use began shortly after 1900 and continued past 1950. Calcium carbide pellets were placed in a container outside the home, with water piped to the container and allowed to drip on the pellets releasing acetylene. This gas was piped to lighting fixtures inside the house, where it was burned, creating a very bright flame. Carbide lighting was inexpensive but was prone to gas leaks and explosions.
Early models of the automobile, car, motorbike and bicycle used carbide lamps as headlamps. Acetylene gas, derived from carbide, enabled early automobiles to drive safely at night. Thick concave mirrors combined with magnifying lenses projected the acetylene flame light. These type of lights were used until reliable batteries and dynamos became available, and manufacturers switched to electric lights.
Acetylene lamps were also used on riverboats for night navigation. The National Museum of Australia has a lamp made in about 1910 that was used on board PS Enterprise, a paddle steamer which has been restored to working order and also in the museum's collection.
They are also used for night hunting.
Caving
| This section possibly contains original research. Please improve it by verifying the claims made and adding inline citations. Statements consisting only of original research should be removed. (February 2022) (Learn how and when to remove this message) |



Early caving enthusiasts, not yet having the advantage of light-weight electrical illumination, introduced the carbide lamp to their hobby. While increasingly replaced by more modern choices, a substantial percentage of cavers still use this method. Many cavers favour carbide lamps for their durability and quality of illumination. They were once favoured for their relative illumination per mass of fuel compared to battery powered devices. Before the advent of high-intensity LED illumination with lithium ion batteries, carbide also had two important advantages over the alternative of miners electric lamps. Miners lamps were intended to last for the duration of a standard working shift, whilst major caving explorations could be much longer, so the carbide could be replenished during the trip. Expeditions involving camping over several days in remote regions might not have access to electricity for recharging. Lamps used in such circumstances would consist of a belt mounted gas generator linked by flexible pipe to a headset.
The acetylene producing reaction is exothermic, which means that the lamp's reactor vessel will become quite warm to the touch; this can be used to warm the hands. The heat from the flame can also be used to warm the body by allowing the exhaust gases to flow under a shirt or poncho pulled out from the body, a technique discovered almost immediately by cold miners, and nicknamed by cavers the "Palmer furnace".
The lamps are sometimes called "stinkies" because of their odour.
Glare reduction
Small carbide lamps called "carbide candles" or "smokers" are used for blackening rifle sights to reduce glare. They are sometimes referred to as "smokers" because of the sooty flame produced by acetylene.
See also
- Dalén light – Automatic controlled acetylene gas lighthouse
- Flashlight – Portable hand-held electric light
- Trouble light – Type of lamp
Notes
- Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. ISBN 0-07-049439-8.
- Morehead, J. T. and de Chalmot, G. (1896). "The Manufacture of Calcium Carbide". Journal of the American Chemical Society. 18 (4): 311–331. doi:10.1021/ja02090a001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Freeman, Horace (1919). "Manufacture of Cyanamide". The Chemical News and the Journal of Physical Science. 117: 232.
- U.S. patent 656,874
- U.S. patent 711,871
- For example on March 10, 1925 Andrew Prader of Spokane, Washington was granted U.S. patent 1,528,848
- Fifty-First Annual Coal Report of Illinois, 1932, Department of Mines and Minerals. Journal Printing Co.: Springfield, ILL., 1933
- Matthews, C. E. (1996). An illuminating reaction. The Science Teacher, 63(5), 30.
- "Chemical characteristics of calcium carbide and its reaction with water". MEL Science. Retrieved 2018-05-16.
- "Paddle Steamer Enterprise, National Museum of Australia". Archived from the original on 2018-09-23. Retrieved 2012-01-30.
- Caving equipment and culture (from Te Ara: The Encyclopedia of New Zealand)
- "Caver's Slang". www-sop.inria.fr.
- Using the Super Smoker. ray-vin.com. Retrieved 11 October 2015.
References
- Clemmer, Gregg (1987). American Miners' Carbide Lamps: A Collectors Guide to American Carbide Mine Lighting. Westernlore Publications. ISBN 978-0870260643.
- Pohs, Henry (1995). The Miners Flame Light Book. Flame Publishing. ISBN 978-0964116504.
- Card, Peter W. (October 2004). Early Vehicle Lighting. Shire Publications. ISBN 978-0-7478-0585-4.
- Thorpe, Dave (2005). Carbide Light: The Last Flame in American Mines. Bergamot Publishing. ISBN 978-0976090526.
External links
- acethylene.com A comprehensive guide to the care and maintenance of acetylene gas lamps
- A User's Guide to Carbide Cap Lamps. Has many good pictures & videos.
- Carbide lamp Demonstration experiment: Instruction and video
- The Carbide Caver A website on the history, restoration, and use of carbide lamps for caving.
| Lighting | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concepts | |||||||||||||
| Methods of generation |
| ||||||||||||
| Stationary | |||||||||||||
| Portable | |||||||||||||
| Automotive | |||||||||||||
| |||||||||||||
| |||||||||||||
| |||||||||||||
| Related topics | |||||||||||||
| Mining equipment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Excavation |
| ||||||||
| Transport |
| ||||||||
| Safety | |||||||||