| Revision as of 20:58, 15 December 2023 edit2601:602:8d00:5ab8:ad37:734b:a409:f1ab (talk) removed superflous 'the'← Previous edit | Revision as of 03:12, 28 January 2024 edit undoJeremyb-phone (talk | contribs)Event coordinators, Extended confirmed users, IP block exemptions, Pending changes reviewers3,739 editsm →Fabric softeners: WP:REFPUNCTags: Mobile edit Mobile app edit Android app editNext edit → | ||
| Line 18: | Line 18: | ||
| Early cotton softeners were typically based on a water ] of ] and ], ], or ] oil.{{citation needed|date=May 2015}} Softening compounds differ in affinity to various fabrics. Some work better on ]-based fibers (i.e., cotton), others have higher affinity to hydrophobic materials like ], ], ], etc. New ]-based compounds, such as ], work by lubricating the fibers. Manufacturers use derivatives with amine- or amide-containing functional groups as well. These groups improve the softener's binding to fabrics. | Early cotton softeners were typically based on a water ] of ] and ], ], or ] oil.{{citation needed|date=May 2015}} Softening compounds differ in affinity to various fabrics. Some work better on ]-based fibers (i.e., cotton), others have higher affinity to hydrophobic materials like ], ], ], etc. New ]-based compounds, such as ], work by lubricating the fibers. Manufacturers use derivatives with amine- or amide-containing functional groups as well. These groups improve the softener's binding to fabrics. | ||
| As softeners are often hydrophobic, they commonly occur in the form of an ].<ref>{{Cite book|last1=Kumar|first1=Asim|last2=Choudhury|first2=Roy|date=2017|title=Principles of Textile Finishing|chapter=6 - Softening |chapter-url=https://www.sciencedirect.com/science/article/pii/B9780081006467000060|publisher=Woodhead Publishing Series in Textiles|pages=109–148|doi=10.1016/B978-0-08-100646-7.00006-0 |isbn=9780081006467 |via=Science Direct}}</ref> In the early formulations, manufacturers used ]s as ]s. The emulsions are usually opaque, milky fluids. However, there are also ]s, where the droplets of the hydrophobic phase are substantially smaller{{Nonspecific|date=August 2011}} |
As softeners are often hydrophobic, they commonly occur in the form of an ].<ref>{{Cite book|last1=Kumar|first1=Asim|last2=Choudhury|first2=Roy|date=2017|title=Principles of Textile Finishing|chapter=6 - Softening |chapter-url=https://www.sciencedirect.com/science/article/pii/B9780081006467000060|publisher=Woodhead Publishing Series in Textiles|pages=109–148|doi=10.1016/B978-0-08-100646-7.00006-0 |isbn=9780081006467 |via=Science Direct}}</ref> In the early formulations, manufacturers used ]s as ]s. The emulsions are usually opaque, milky fluids. However, there are also ]s, where the droplets of the hydrophobic phase are substantially smaller.{{Nonspecific|date=August 2011}} Microemulsions provide the advantage of increased ability of smaller particles to penetrate into the fibers. Manufacturers often use a mixture of cationic and non-ionic surfactants as an emulsifier. Another approach is a polymeric network, an emulsion polymer. | ||
| In addition to fabric softening chemicals, fabric softeners may include acids or bases to maintain optimal ] for absorption, silicone-based ]s, emulsion stabilizers, fragrances, and colors. | In addition to fabric softening chemicals, fabric softeners may include acids or bases to maintain optimal ] for absorption, silicone-based ]s, emulsion stabilizers, fragrances, and colors. | ||
Revision as of 03:12, 28 January 2024
Chemical compoundA fabric softener (American English) or fabric conditioner (British English) is a conditioner that is applied to laundry after it has been washed in a washing machine. A similar, more dilute preparation meant to be applied to dry fabric is known as a wrinkle releaser.
A fabric softener reduces the harsh feel of items that were dried in open-air, adds a fragrance to the laundry, and/or imparts anti-static properties to textiles. In contrast to laundry detergents, fabric softeners may be regarded as a kind of after-treatment laundry aid.
Fabric softeners are either in the form of a liquid that is typically added during the washing machine rinse cycle, or as a dryer sheet which is added to a tumble dryer before drying begins. Liquid fabric softeners may be added manually during the rinse cycle, automatically if the machine has a dispenser designed for this purpose, through use of a dispensing ball, or poured onto a piece of laundry to be dried (such as a wash cloth) which is then put into the dryer.
Washing machines put great mechanical stress on textiles, particularly natural fibers such as cotton and wool. The fibers at the fabric's surface are squashed and frayed, and this condition hardens into place when drying the laundry in open-air, giving the textiles a harsh feel. Using a tumble dryer results in a softening effect, but it is less than what can be achieved through the use of a fabric softener.
As of 2009, nearly 80% of US households had a mechanical clothes dryer. As such, fabric softeners are primarily used there to impart anti-static properties and/or a fragrance to laundry.
Mechanism of action
Fabric softeners coat the surface of a fabric with chemical compounds that are electrically charged, causing threads to "stand up" from the surface and thereby imparting a softer and fluffier texture. Cationic softeners bind by electrostatic attraction to the negatively charged groups on the surface of the fibers and neutralize their charge. The long aliphatic chains then line up towards the outside of the fiber, imparting lubricity.
Fabric softeners impart anti-static properties to fabrics, and thus prevent the build-up of electrostatic charges on synthetic fibers, which in turn eliminates fabric cling during handling and wearing, crackling noises, and dust attraction. Also, fabric softeners make fabrics easier to iron and help reduce wrinkles in garments. In addition, they reduce drying times so that energy is saved when softened laundry is tumble-dried. Additionally, they can also impart a pleasant fragrance to the laundry.
Fabric softeners
Early cotton softeners were typically based on a water emulsion of soap and olive oil, corn oil, or tallow oil. Softening compounds differ in affinity to various fabrics. Some work better on cellulose-based fibers (i.e., cotton), others have higher affinity to hydrophobic materials like nylon, polyethylene terephthalate, polyacrylonitrile, etc. New silicone-based compounds, such as polydimethylsiloxane, work by lubricating the fibers. Manufacturers use derivatives with amine- or amide-containing functional groups as well. These groups improve the softener's binding to fabrics.
As softeners are often hydrophobic, they commonly occur in the form of an emulsion. In the early formulations, manufacturers used soaps as emulsifiers. The emulsions are usually opaque, milky fluids. However, there are also microemulsions, where the droplets of the hydrophobic phase are substantially smaller. Microemulsions provide the advantage of increased ability of smaller particles to penetrate into the fibers. Manufacturers often use a mixture of cationic and non-ionic surfactants as an emulsifier. Another approach is a polymeric network, an emulsion polymer.
In addition to fabric softening chemicals, fabric softeners may include acids or bases to maintain optimal pH for absorption, silicone-based anti-foaming agents, emulsion stabilizers, fragrances, and colors.
Cationic fabric softeners
Rinse-cycle softeners usually contain cationic surfactants of the quaternary ammonium type as the main active ingredient. Cationic surfactants adhere well to natural fibers (wool, cotton), but less so to synthetic fibers. Cationic softeners are incompatible with anionic surfactants in detergents because they combine with them to form a solid precipitate. This requires that the softener be added in the rinse cycle. Fabric softener reduces the absorbency of textiles, which adversely affects the function of towels and microfiber cloth.
Formerly, the active material of most softeners in Europe, the United States, and Japan, was distearyldimethylammonium chloride (DSDMAC) or related quat salts. Due to their poor biodegradability, such tallow-derived compounds were replaced by the more labile ester-quats in the 1980s and 1990s.
Conventional softeners, which contain 4–6% active material, have been partially replaced in many countries by softener concentrates having some 12–30% active material.
- Cationic surfactants used as fabric softeners
-
 Diethyl ester dimethyl ammonium chloride (DEEDMAC)
Diethyl ester dimethyl ammonium chloride (DEEDMAC)
-
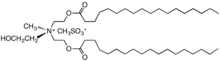 TEAQ (triethanolamine quat)
TEAQ (triethanolamine quat)
-
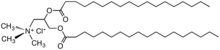 HEQ (Hamburg esterquat)
HEQ (Hamburg esterquat)
-
 Distearyldimethylammonium chloride (DSDMAC)
Distearyldimethylammonium chloride (DSDMAC)
Anionic fabric softeners
Anionic softeners and antistatic agents can be, for example, salts of monoesters and diesters of phosphoric acid and the fatty alcohols. These are often used together with the conventional cationic softeners. Cationic softeners are incompatible with anionic surfactants in detergents because they combine with them to form a solid precipitate. This requires that they be added in the rinse cycle. Anionic softeners can combine with anionic surfactants directly. Other anionic softeners can be based on smectite clays. Some compounds, such as ethoxylated phosphate esters, have softening, anti-static, and surfactant properties.
Risks
As with soaps and detergents, fabric softeners may cause irritant dermatitis. Manufacturers produce some fabric softeners without dyes and perfumes to reduce the risk of skin irritation. Fabric softener overuse may make clothes more flammable, due to the fat-based nature of most softeners. Some deaths have been attributed to this phenomenon, and fabric softener makers recommend not using them on clothes labeled as flame-resistant.
Additional reading
- Terlep, Sharon (16 December 2016). "Millennials Are Fine Without Fabric Softener; P&G Looks to Fix That". Wall Street Journal. Retrieved 17 December 2016.
References
- ^ Eduard Smulders; Eric Sung (2012). "Laundry Detergents, 2. Ingredients and Products". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o15_013. ISBN 978-3527306732.
- Jones, C. R.; Corona, A.; Amador, C.; Fryer, P. J. (2022-07-15). "Dynamics of fabric and dryer sheet motion in domestic clothes dryers". Drying Technology. 40 (10): 2087–2104. doi:10.1080/07373937.2021.1918706. ISSN 0737-3937. S2CID 236596597.
- Eduard Smulders; Wolfgang Rybinski; Eric Sung; Wilfried Rähse; Josef Steber; Frederike Wiebel; Anette Nordskog (2007). "Laundry Detergents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 86–87. doi:10.1002/14356007.a08_315.pub2. ISBN 978-3527306732.
- "ENERGY STAR Market & Industry Scoping Report: Dryers" (PDF). Energy Star. United States Government. p. 3. Retrieved 15 December 2023.
- Kumar, Asim; Choudhury, Roy (2017). "6 - Softening". Principles of Textile Finishing. Woodhead Publishing Series in Textiles. pp. 109–148. doi:10.1016/B978-0-08-100646-7.00006-0. ISBN 9780081006467 – via Science Direct.
- Wang, Linda (14 Apr 2008). "Dryer Sheets — The science that gives clothing a soft feel and fresh scent as it prevents static cling". Chemical and Engineering News. 86 (15): 47. ISSN 0009-2347.
- Morrison, Chris (2009-11-30). "How Dryer Sheets Work". HowStuffWorks. Retrieved 2021-01-17.
- "Fabric softener and anti-static compositions – Patent 4118327". Freepatentsonline.com. 1977-03-28. Retrieved 2009-06-04.
- "Contact dermatitis". Medline. Retrieved 2015-10-24.
- "Liquid fabric softener may make clothes more flammable: Quebec coroner". CBC. Retrieved 2015-11-20.
- Heloise (2001-11-28). "Cleaning Flame-Retardant Clothing". Good Housekeeping. Retrieved 2020-12-06.
| Laundry | |
|---|---|
| List of laundry topics | |
| Chemicals | |
| Washing | |
| Drying | |
| Folding | |
| Finishing | |
| Concepts | |
| Organizations | |
| Culture | |
| Accessories | |
| Law | |
| Places | |