| Revision as of 05:08, 19 April 2024 edit2409:40e2:b:c9db:8000:: (talk)No edit summaryTags: Visual edit Mobile edit Mobile web edit← Previous edit | Latest revision as of 08:52, 27 June 2024 edit undoUltimateChance (talk | contribs)261 editsmNo edit summaryTag: Visual edit | ||
| Line 62: | Line 62: | ||
| ==Aromaticity== | ==Aromaticity== | ||
| Notably, annulene is the first annulene after ] (annulene) to be fully ]: its π-system contains 4n + 2 electrons (n = 4), and it is large enough to comfortably accommodate six hydrogen atoms in its interior, allowing it to adopt a planar shape, thus satisfying ]. The discovery of aromatic stabilization for annulene is historically significant for confirming earlier theoretical predictions based on ], since simple versions of valence bond theory did not readily explain |
Notably, annulene is the first annulene after ] (annulene) to be fully ]: its π-system contains 4n + 2 electrons (n = 4), and it is large enough to comfortably accommodate six hydrogen atoms in its interior, allowing it to adopt a planar shape, thus satisfying ]. The discovery of aromatic stabilization for annulene is historically significant for confirming earlier theoretical predictions based on ], since simple versions of valence bond theory did not readily explain 4''n'' + 2 rule. | ||
| The <sup>1</sup>H ] of this compound exhibits |
The <sup>1</sup>H ] of this compound exhibits hallmarks of a system with an ], with the 12H signal of exterior hydrogens at 9.25 ppm, while 6H signal of interior hydrogens resonates at a remarkable −2.9 ppm in THF-''d<sub>8</sub>'' at −60 °C. On the other hand, a single signal at 5.45 ppm (weighted average of two individual signals) is observed at 120 °C. This is consistent with rapid exchange of exterior and interior hydrogens at that temperature. The ] in annulene are in between those of single and double carbon–carbon bond, with two bond lengths observed ]: 138.9 pm (concave edges) and 140.7 pm (convex edges) and are indicative of significant ]. The favorability of delocalization is, in turn, interpreted as evidence for aromaticity. For comparison, these values are close to bond length of benzene (140 pm).<ref name="Jux2016">{{Cite journal|last1=Jux|first1=Norbert|last2=R. Schleyer|first2=Paul v|last3=Majetich|first3=George|last4=Meyer|first4=Karsten|last5=Hampel|first5=Frank|last6=W. Heinemann|first6=Frank|last7=V. Nizovtsev|first7=Alexey|last8=Lungerich|first8=Dominik|date=2016|title=Annulene put into a new perspective|journal=Chemical Communications|language=en|volume=52|issue=25|pages=4710–4713|doi=10.1039/C6CC01309K|pmid=26953607|doi-access=free}}</ref> | ||
| annulene are around 139 to 141 pm, indicating delocalization and a bond order between 1 and 2. In general, carbon-carbon single bonds and double bonds are 154 pm and 134 pm, respectively, while benzene, with a bond order of 1.5, the bond-length is 140 pm.]] | annulene are around 139 to 141 pm, indicating delocalization and a bond order between 1 and 2. In general, carbon-carbon single bonds and double bonds are 154 pm and 134 pm, respectively, while benzene, with a bond order of 1.5, the bond-length is 140 pm.]] | ||
| Based on |
Based on enthalpy of hydrogenation, overall ] energy has been estimated to be 37 kcal/mol.<ref>{{Cite journal|date=1974-11-06|title=The Stabilization Energy of Annulene. A thermochemical determination|journal=Helvetica Chimica Acta|volume=57|issue=7|pages=2276–2288|doi=10.1002/hlca.19740570745|issn=0018-019X|last1=Oth|first1=Jean F. M.|last2=Bünzli|first2=Jean-Claude|last3=De Julien De Zélicourt|first3=Yves}}</ref> This is about the same as that of benzene; however, this energy is spread out over 18 atoms instead of 6, so annulene experiences a weaker stabilization than benzene. In terms of reactivity, it is somewhat more air- and light-stable than annulene]] and annulene]], which are, respectively, weakly aromatic and nonaromatic due to transannular interactions. Nevertheless, it rapidly undergoes ]s, much like other ], and attempts to effect ] on annulene failed.<ref>{{cite journal|author1=Sondheimer, F.|author2=Wolovsky, R. |author3=Amiel, Y.|year=1962|title=Unsaturated Macrocyclic Compounds. XXIII. The Synthesis of the Fully Conjugated Macrocyclic Polyenes Cyclooctadecanonaene (Annulene), Cyclotetracosadodecaene (Annulene), and Cyclotriacontapentadecaene (Annulene).|journal=]|volume=68|issue=2|pages=274–284|doi=10.1021/ja00861a030}}</ref> | ||
| Despite the usual interpretation of annulene as an 18-electron aromatic system, a 2014 theoretical study suggested that annulene may be thought of as having only three completely delocalized ] associated with its aromaticity, while |
Despite the usual interpretation of annulene as an 18-electron aromatic system, a 2014 theoretical study suggested that annulene may be thought of as having only three completely delocalized ] associated with its aromaticity, while other six π bonds represent conjugated ] ("3c-2e") π bonds on periphery of molecule.<ref>{{cite journal|last=Ivanov|first=A.|author2=Boldyrev. A|title=Deciphering aromaticity in porphyrinoids via adaptive natural density partitioning|journal=Org. Biomol. Chem.|volume=12|issue=32|pages=6145–6150|year=2014|doi=10.1039/C4OB01018C|pmid=25002069}}</ref> | ||
| ==Synthesis== | ==Synthesis== | ||
| The compound was first synthesised by ].<ref>In the literature and some internet references, Sondheimer is sometimes misspelled as Sandheimer.</ref> |
The compound was first synthesised by ].<ref>In the literature and some internet references, Sondheimer is sometimes misspelled as Sandheimer.</ref> The original synthesis started by ] of di-] 1,5-hexadiyne with ] in ] to give the trimer, followed by ] and ] with ] in ] and was concluded with ] ] with ].<ref>{{OrgSynth | title = <nowiki>Annulene</nowiki> | author1 = Stöckel, K. |author2=Sondheimer, F. | collvol = 6 | collvolpages = 68 | year = 1988 | prep = cv6p0068}}</ref> | ||
| ==See also== | ==See also== | ||
Latest revision as of 08:52, 27 June 2024
| |||
 "Herringbone" crystal structure of annulene | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (1Z,3E,5E,7Z,9E,11E,13Z,15E,17E)-Cyclooctadeca-1,3,5,7,9,11,13,15,17-nonaene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C18H18 | ||
| Molar mass | 234.3 g·mol | ||
| Appearance | red-brown crystals | ||
| Density | 1.134 g/cm (calc.) | ||
| Structure | |||
| Space group | monoclinic, P21/a | ||
| Lattice constant | a = 1.4984 (5) nm, b = 0.4802(2) nm, c = 1.0260(3) nmα = 90°, β = 111.52(1)°, γ = 90° | ||
| Formula units (Z) | 2 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
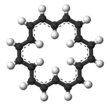
Cyclooctadecanonaene or annulene is an organic compound with chemical formula C
18H
18. It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis,trans,trans,cis,trans,trans,cis,trans,trans configuration. It is reported to be a red-brown crystalline solid.
Aromaticity
Notably, annulene is the first annulene after benzene (annulene) to be fully aromatic: its π-system contains 4n + 2 electrons (n = 4), and it is large enough to comfortably accommodate six hydrogen atoms in its interior, allowing it to adopt a planar shape, thus satisfying Hückel's rule. The discovery of aromatic stabilization for annulene is historically significant for confirming earlier theoretical predictions based on molecular orbital theory, since simple versions of valence bond theory did not readily explain 4n + 2 rule.
The H NMR of this compound exhibits hallmarks of a system with an aromatic ring current, with the 12H signal of exterior hydrogens at 9.25 ppm, while 6H signal of interior hydrogens resonates at a remarkable −2.9 ppm in THF-d8 at −60 °C. On the other hand, a single signal at 5.45 ppm (weighted average of two individual signals) is observed at 120 °C. This is consistent with rapid exchange of exterior and interior hydrogens at that temperature. The bond lengths in annulene are in between those of single and double carbon–carbon bond, with two bond lengths observed crystallographically: 138.9 pm (concave edges) and 140.7 pm (convex edges) and are indicative of significant delocalization. The favorability of delocalization is, in turn, interpreted as evidence for aromaticity. For comparison, these values are close to bond length of benzene (140 pm).

Based on enthalpy of hydrogenation, overall resonance energy has been estimated to be 37 kcal/mol. This is about the same as that of benzene; however, this energy is spread out over 18 atoms instead of 6, so annulene experiences a weaker stabilization than benzene. In terms of reactivity, it is somewhat more air- and light-stable than annulene and annulene, which are, respectively, weakly aromatic and nonaromatic due to transannular interactions. Nevertheless, it rapidly undergoes electrophilic additions, much like other polyenes, and attempts to effect Friedel-Crafts type reactions on annulene failed.
Despite the usual interpretation of annulene as an 18-electron aromatic system, a 2014 theoretical study suggested that annulene may be thought of as having only three completely delocalized π bonds associated with its aromaticity, while other six π bonds represent conjugated three-center-two-electron ("3c-2e") π bonds on periphery of molecule.
Synthesis
The compound was first synthesised by Franz Sondheimer. The original synthesis started by Eglinton reaction of di-alkyne 1,5-hexadiyne with copper(II) acetate in pyridine to give the trimer, followed by deprotonation and isomerization with potassium tert-butoxide in tert-butanol and was concluded with hydrogen organic reduction with Lindlar catalyst.
See also
References
- ^ Gorter, S.; Rutten-Keulemans, E.; Krever, M.; Romers, C.; Cruickshank, D. W. J. (1995). "[18]-Annulene, C18H18, structure, disorder and Hückel's 4 n + 2 rule". Acta Crystallographica Section B Structural Science. 51 (6): 1036–1045. Bibcode:1995AcCrB..51.1036G. doi:10.1107/S0108768195004927.
- Jux, Norbert; R. Schleyer, Paul v; Majetich, George; Meyer, Karsten; Hampel, Frank; W. Heinemann, Frank; V. Nizovtsev, Alexey; Lungerich, Dominik (2016). "[18]Annulene put into a new perspective". Chemical Communications. 52 (25): 4710–4713. doi:10.1039/C6CC01309K. PMID 26953607.
- Oth, Jean F. M.; Bünzli, Jean-Claude; De Julien De Zélicourt, Yves (1974-11-06). "The Stabilization Energy of Annulene. A thermochemical determination". Helvetica Chimica Acta. 57 (7): 2276–2288. doi:10.1002/hlca.19740570745. ISSN 0018-019X.
- Sondheimer, F.; Wolovsky, R.; Amiel, Y. (1962). "Unsaturated Macrocyclic Compounds. XXIII. The Synthesis of the Fully Conjugated Macrocyclic Polyenes Cyclooctadecanonaene (Annulene), Cyclotetracosadodecaene (Annulene), and Cyclotriacontapentadecaene (Annulene)". J. Am. Chem. Soc. 68 (2): 274–284. doi:10.1021/ja00861a030.
- Ivanov, A.; Boldyrev. A (2014). "Deciphering aromaticity in porphyrinoids via adaptive natural density partitioning". Org. Biomol. Chem. 12 (32): 6145–6150. doi:10.1039/C4OB01018C. PMID 25002069.
- In the literature and some internet references, Sondheimer is sometimes misspelled as Sandheimer.
- Stöckel, K.; Sondheimer, F. (1988). "Annulene". Organic Syntheses; Collected Volumes, vol. 6, p. 68.
| Annulenes | |
|---|---|
| Even–numbered | |
| Odd–numbered | |
| Compounds in italics are aromatic | |