| Revision as of 09:27, 26 October 2008 editJJ Harrison (talk | contribs)Extended confirmed users11,943 edits →Animation: new section← Previous edit | Revision as of 15:57, 27 October 2008 edit undoජපස (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers60,451 edits →Animation: helpNext edit → | ||
| Line 325: | Line 325: | ||
| I found particularly useful in explaining the basics behind free-radical polymerisation and a free version for wikipedia would be a very useful addition to the relevant article if anyone has the time and knowhow. ] (]) 09:27, 26 October 2008 (UTC) | I found particularly useful in explaining the basics behind free-radical polymerisation and a free version for wikipedia would be a very useful addition to the relevant article if anyone has the time and knowhow. ] (]) 09:27, 26 October 2008 (UTC) | ||
| ==Cold fusion== | |||
| HELP! We have Cold Fusion proponents dramatically asserting ] over ]. I need all the help I can get. ] (]) 15:57, 27 October 2008 (UTC) | |||
Revision as of 15:57, 27 October 2008
| Chemistry Project‑class | |||||||
| |||||||
Discussion of the WikiProject Chemistry - Please add your comment and discussion here. Older discussions are archived.
This discussion page is about the Chemistry project itself, for detailed, in-depth discussions about specific topics, you'd be best served at the talk page of the specific subject, e.g., Chemicals, Chemical infoboxes, etc. There is also an image request page which might be of interest to you.
|
Archives |
|
Commons:Commons:Administrators/Requests and votes/Edgar181
There appear to be no chemist-admins on commons. Maybe you guys might have something to say? --Rifleman 82 (talk) 18:28, 20 August 2008 (UTC)
- Thanks for the support everyone. If there are any chemistry-related administrative actions needed on Commons, such as deleting incorrect chemical structure diagrams, please just let me know. -- Ed (Edgar181) 13:29, 1 September 2008 (UTC)
Infobox reaction?
Hey guys
What do you think of User:Rifleman_82/Petasis_reaction? I think it would be helpful for having a box which gives, at a glance, the gist of a particular type of reaction? I need a bit of help with the ifexist operator, but this is a start? --Rifleman 82 (talk) 20:38, 20 August 2008 (UTC)
User:Rifleman_82/Heck reaction is another test example. Ultimately, I hope that we are able to use this template to categorize (= make searchable) reactions by substrate, product, catalyst, etc. --Rifleman 82 (talk) 20:51, 20 August 2008 (UTC)
New policy proposal and draft help
Misplaced Pages:Scientific standards
I have drafted a new proposal and would like help in clarifying, adjusting, adapting, and improving it. It is based on five years of work here at Misplaced Pages (not always the prettiest, I might add). I think it summarizes the opinions of a great majority of editors as to how to handle scientific situations. This proposal serves as a nexus between WP:NPOV and WP:RS for cases where we are dealing with observable reality. It is needed because there are a lot of editors who don't seem to understand what entails best-practices when writing a reliable reference work about observable reality. I don't pretend that this version is perfect, and would appreciate any and all additions, suggestions people may have for getting to some well-regarded scientific standards.
Note that these standards would apply only when discussing matters directly related to observable reality. These standards are inspired in part by WP:SPOV but avoid some of the major pitfalls of that particular proposal. In particular, the idea that SPOV even exists is a real problem. However, I think it is undeniable that we should have some standards for writing about scientific topics.
See also WP:SCI for another failed proposal that dovetails with this one. I hope this particular proposal is more in-line with the hole I see in policy/guidelines for dealing with these situations.ScienceApologist (talk) 19:55, 19 August 2008 (UTC)
The above proposal was originally placed on the Physics Project Talk page, but has been added on other projects but not here. I think there should be input from chemists. --Bduke (talk) 22:14, 20 August 2008 (UTC)
PR page
i think it should be labeled as historical since nobody uses it anymore. Nergaal (talk) 19:24, 29 August 2008 (UTC)
WikiProject Explosives
Hello, WikiProject Explosives has been launched; feel free to sign up and pitch in if you wish. EVCM (talk) 22:16, 29 August 2008 (UTC)
Template:Atomic models
I created this template so people could quickly-browse atomic models. The skeleton is there, but little meat. Help is appreciated. Headbomb {ταλκ – WP Physics: PotW} 01:29, 1 September 2008 (UTC)
Elements report
Greetings, friends! I am Cryptic, a member of Misplaced Pages:WikiProject Elements. I am pleased to announce the first edition of my elements report. For those of you who have collaborated with us before (Stone and Itub, among others) or those who wish to lend a hand for the first time, this report is a good way to figure out exactly which areas need our efforts the most. Plus, if you praise me enough, I may be inclined to do a report for you guys, too! --Cryptic C62 · Talk 01:00, 3 September 2008 (UTC)
- Nice! How automatic is the report generation? I ask because while it is straightforward to constrain the elements report to 118 articles on the elements, there's no such natural constraint for chemistry or for chemicals, and the number of articles would probably be much larger. I suppose you could constrain it to articles that are currently rated Top and High importance (about 100 and 200 articles, respectively), but that would defeat part of the purpose, which is to identify important but under-appreciated articles. --Itub (talk) 08:58, 3 September 2008 (UTC)
- Sadly, I did it all myself using stats.grok.se and MS Excel; there was nothing automatic about it. As for a chemistry report, what if I restricted it to the first 3 pages of Misplaced Pages:Version 1.0 Editorial Team/Chemistry articles by quality ? That would include 1203 assessed articles and lists and leave out the hundreds of unassessed articles. Or, to make it easier, I could then leave out biographical articles, such as Adolf von Baeyer and Nikolay Semyonov. Even without biographies though, that would take quite a while. Might need the help of a bot...? --Cryptic C62 · Talk 00:04, 5 September 2008 (UTC)
- I agree, this is very nice. You may also be interested in our Misplaced Pages 1.0 stats, for which we just generated our first "good" set of elements data using SelectionBot. There are similar results for chemistry and chemicals:
- Main index
- Chemical elements
- Chemistry
- Chemicals (big file, slower to load)
Perhaps these data are useful? They are generated by a bot, and placed on the Toolserver. FYI, the articles in bold are the ones that will (as a minimum) go on the next DVD; in fact, ALL known elements are already tagged to be in (for completeness). Walkerma (talk) 21:17, 4 September 2008 (UTC)
- Hrm. I had never seen that before. Perhaps I'll use that for publishing the next edition of the elements report using August's page view data. That's the most time-consuming part; updating the graphics is a piece of cake. --Cryptic C62 · Talk 00:04, 5 September 2008 (UTC)
- I see that Chemical reaction is of "unknown" importance to this project… just nipping off to fix that ;) Physchim62 (talk) 23:23, 4 September 2008 (UTC)
Chemistry Portals
I just came across Portal:Physical chemistry and then Portal:Analytical chemistry and Portal:Organic chemistry. All three were created by the same editor, User:Chaos last December and they have had no other edits since then. After that he created Portal:Pharmacy and Pharmacology, but that one has developed. All three, to not mince words, are a total mess. I did a few minor changes to the first one, that being my area, as I explored what is there. We do not seem to have noticed these. Can we bring them up to speed or should we just get rid of them? --Bduke (talk) 08:09, 4 September 2008 (UTC)
Stats.grok.se reports the following pagehits for the month of August 2008:
- Portal:Physical chemistry - 417
- Portal:Analytical chemistry - 853
- Portal:Organic chemistry - 1285
Pagehits show that these portals are not very important (c.f. HCl which got 62k hits). However, they might be nice to have as a "content page" of our articles. The portals, as they stand, are pretty, but if we don't intend to update them much, they should remain more static for maintenance reasons. Perhaps we should fold them all into a Portal:Chemistry? Have a one-para blurb on the essence of a certain discipline, the key concepts (5-10), one picture with something interesting to catch the eye, and that's it? If you guys are interested, I can do that. --Rifleman 82 (talk) 15:58, 5 September 2008 (UTC)
Sorry :):):)
Sorry to anyone who felt compelled to revert my changes. It's just that I'm in my last year of Chemistry, and I had a small bit of trouble with understanding the structural formulas of Alkenes and the like, so I wanted everyone else to understand it without difficulty. Once again, my sincere apologies to everyone.
-Peace Out!-
mÆniac 17:11, 6 September 2008 (UTC)
- No need to excuse :-) I have made flat connectivity structures (Lewis structures?) for some simple linear molecules as they are indeed easier to understand for beginners (even if they are quite misleading with regards to three-dimensional structure). Feel free to add them to the respective articles (but please do not replace existing skeletal formulas):
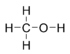
|
|||
| Flat connectivity structure of acetaldehyde | |||
| Flat connectivity structure of acetic acid |
Lithium sulphur battery
I was surprised that we didn't have an article on the lithium sulfur battery after reading that it had been used to set a record for the longest solar-powered flight. I created a stub, but it needs expert attention from one or more chemists. Can you help with it please? Neut Nuttinbutter (talk) 13:53, 7 September 2008 (UTC)
IRC meeting
I'll be around today at 1600 UTC at channel #wikichem, as I proposed last week, for anyone who wants to discuss Beetstra's bot. We do need to figure out the best way to coordinate the upload of validated data. Walkerma (talk) 15:34, 9 September 2008 (UTC)
nomenclature
As WIKIPEDIA is increasingly used by college and graduate students Chemistry, it would be great of the chemistry sites of wikipedia would at least (also) mention the correct names of chemical compounds in the wiki, i.e. the names that IUPAC (International Union of Pure and Applied Chemistry) recommends to use. This would help enormously to help communication, and let students who consult wikipedia use correct names. Reedijk (talk) 12:33, 10 September 2008 (UTC)
- Yes, that is true. But wikipedia is also used by non-chemists a lot, and therefore it was chosen here to use 'common names' in stead of the correct names (though it should be good practice to redirect all other names to the correct article.
- E.g., Osmium tetroxide is the name that is the name we use here, as it is the 'common name'; Osmium tetraoxide is a redirect to the former one, and linking to both works. More info in Misplaced Pages:WikiProject Chemicals/Style guidelines. --Dirk Beetstra 12:48, 10 September 2008 (UTC)
- But chemicals articles also give (or should give) the IUPAC nomenclature even if the common name is chosen for the article title. --Itub (talk) 12:57, 10 September 2008 (UTC)
- Actually, that happens (or should happen) in the
{{chembox new}}. --Dirk Beetstra 13:04, 10 September 2008 (UTC)- If you want to appreciate the world order that Reedijk seeks, then look at the edits on Tetrafluoroborate. We live in a complicated world, and many nomenclatures are currently evolving and devolving. The chem boxes should list alternative names, including the IUPAC ones, but we dont want to follow Reedijk's example. The non-IUPAC names are not simply for non-chemists, practicing chemists adhere only loosely to IUPAC. I am sure that the absence of lock-stepping infuriates them, but that's life. Also I dont think that Misplaced Pages is a vehicle for imposing culture, vs reporting on it. Otherwise we'd would be using IUPAC in Esparanto. But I digress, not having finished my cup of aqueous 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione.--Smokefoot (talk) 13:21, 10 September 2008 (UTC)
We go to quite considerable lengths to ensure that are articles about chemical compounds contain the various IUPAC names – note that there is not a one-to-one correspondence between structure and name – but we're not perfect. After all, even the experts sometimes make mistakes ! Nor would we wish to descend into pedantry or dogmatism: I, for one, am at a loss to see how IUPAC thinks it is "helping communication" by insisting on tetrafluoridoborate while still allowing perchlorate, to give but one example. Physchim62 (talk) 15:29, 10 September 2008 (UTC)
- I don't see what is controversial here. I thought that our policy was always to include the IUPAC name, and indeed I suspect that Smokefoot probably pasted the caffeine one in from our article! All Reedijk is asking is that we "would at least (also) mention" IUPAC names. We should. We have a database that we are going to start uploading (when we get around to it!), and I can reassure Reedijk that EVERY one of these articles will have a validated IUPAC name in it. These are mainly organics, but inorganics will follow. The main problem we have, though, is with pseudo-IUPAC names, where people try to replace the IUPAC approved name (such as acetone) with one their (badly written) textbook thinks is the IUPAC name (say, propan-2-one). But as for true IUPAC names, I say, yes, let's always include it! Walkerma (talk) 15:43, 10 September 2008 (UTC)
Yep, it's helpful to include the IUPAC names of molecules in their articles, but it is not necessary to replace every instance of, say, tetrafluoroborate with tetrafluoridoborate(III). Noting the IUPAC name is one thing, but using it everywhere (e.g. these edits) often makes an article less easy to read. IUPAC names tend not to roll off the tongue! And no-one should be changing the filenames of images, resulting in the disappearance of an image from an article.
Ben (talk) 17:33, 10 September 2008 (UTC)
Talk:Alkaloid
Your comment would be welcome on Talk:Alkaloid, one user has very special ideas on what constitutes an alkaloid. Thanks, Cacycle (talk) 01:04, 12 September 2008 (UTC)
Misplaced Pages 0.7 articles have been selected for Chemistry
Misplaced Pages 0.7 is a collection of English Misplaced Pages articles due to be released on DVD, and available for free download, later this year. The Misplaced Pages:Version 1.0 Editorial Team has made an automated selection of articles for Version 0.7.
We would like to ask you to review the articles selected from this project. These were chosen from the articles with this project's talk page tag, based on the rated importance and quality. If there are any specific articles that should be removed, please let us know at Misplaced Pages talk:Version 0.7. You can also nominate additional articles for release, following the procedure at Misplaced Pages:Release Version Nominations.
A list of selected articles with cleanup tags, sorted by project, is available. The list is automatically updated each hour when it is loaded. Please try to fix any urgent problems in the selected articles. A team of copyeditors has agreed to help with copyediting requests, although you should try to fix simple issues on your own if possible.
We would also appreciate your help in identifying the version of each article that you think we should use, to help avoid vandalism or POV issues. These versions can be recorded at this project's subpage of User:SelectionBot/0.7. We are planning to release the selection for the holiday season, so we ask you to select the revisions before October 20. At that time, we will use an automatic process to identify which version of each article to release, if no version has been manually selected. Thanks! For the Misplaced Pages 1.0 Editorial team, SelectionBot 20:40, 15 September 2008 (UTC)
Category:Articles needing expert attention since January 2007
Almost every article in this category is a chemical under this Wikiproject. It would be very helpful if some knowledgeable could take a look through these.--BirgitteSB 06:09, 19 September 2008 (UTC)
- I think the template can be removed. These articles are simply stubs, and require just about the same amount of expertise as any other chemical article. --Itub (talk) 08:34, 19 September 2008 (UTC)
- Say the word and I'll run chem-awb (talk · contribs) through, removing the tags. --Rifleman 82 (talk) 09:45, 19 September 2008 (UTC)
- Looking VERY quickly through the list tungsten(IV) silicide caught my eye, this compound WSi2 is IMO best called tungsten disilicide to prevent confusion with other W-Si phases. Certainly tungsten(IV) is incorrect. --Axiosaurus (talk) 08:15, 20 September 2008 (UTC)
- The articles are mostly very short stubs along the lines of "Foobarium machinide is a chemical compound." They're often completely unreferenced as well. I think we actually need to do some work on them, rather than simply removing the templates. Physchim62 (talk) 12:14, 20 September 2008 (UTC)
I was looking at some of the yttrium compounds on the list, and it is really hard to come up with anything notable to say about them! For example, YAs has about 30 refs in CAS, but all I can say is that it has been made, and some of its structural, electrical, and thermodynamic properties have been measured. I haven't found any uses or anything remarkable to say. There is one hit in Google Books that talks of a "neodymium/yttrium arsenide garnet laser", but after trying to find more information about such a laser I think they may have confused it with a neodymium/yttrium aluminum garnet laser (it would have the same acronym).
I hate when people nominate chemical articles for deletion saying that they are not notable because they are usually wrong. But is it possible that this may be a real case of a non-notable chemical? --Itub (talk) 09:35, 14 October 2008 (UTC)
Templates Disputed_chem and Low_quality_chem for deletion
The templates that we use to tag erroneous or bad chemical structures here and at the Commons have been listed for deletion, your comment would be welcome on Misplaced Pages:Templates_for_deletion#Chemistry_dispute_templates. Cacycle (talk) 03:23, 20 September 2008 (UTC)
Could someone knowledgable in HPLC check this edit?
Does increasing the polarity of the mobile phase in reverse-phase HPLC increase or decrease retention times? The IP has a history of good-faith contributions. This one either corrected, or created, a serious mistake. Adrian J. Hunter 05:16, 27 September 2008 (UTC)
- It's been a while for me, but as I recall a more polar mobile phase will give a shorter RT in RP-HPLC. I've reverted the edit in question. Yilloslime (t) 06:03, 27 September 2008 (UTC)
- That's my memory as well, but again, it's been a while. It also depends somewhat on the polarity of your analyte… Physchim62 (talk) 10:38, 27 September 2008 (UTC)
- Thanks for the responses. "It also depends somewhat on the polarity of your analyte..." – I'm no expert, but intuitively I'd have thought a more polar mobile phase would decrease the retention time of a highly polar analyte, but increase the retention time of a highly hydrophobic analyte. If there's any doubt, perhaps it would be safest just to delete the sentence in question? Adrian J. Hunter 13:29, 27 September 2008 (UTC)
- That's my memory as well, but again, it's been a while. It also depends somewhat on the polarity of your analyte… Physchim62 (talk) 10:38, 27 September 2008 (UTC)
"Melamine cyanurate" renaming debate
Hi, there's a discussion going on about a recent attempt to rename melamine cyanurate, located on the talk page here. I thought the naming guidelines were pretty straight forward, but I may be wrong. Please have a look. NJGW (talk) 00:05, 28 September 2008 (UTC)
Equivalent (chemistry)
I'm trying to bring some order into our articles on the concept of the equivalent – for the moment, we have equivalent mass, equivalent weight, gram equivalent, molar equivalent and a section on normality in concentration as well as equivalent (chemistry)! I was wondering if anyone could supply an example of the use of equivalent in modern chemistry, as in the sense of "stoichiometric quantity" or similar. I would come up with one myself, but I've been out of the lab for quite a while now… Physchim62 (talk) 10:49, 28 September 2008 (UTC)
Dimsyl sodium?
I refer to Image:Ylideformation.png. Is this structure (bottom right) the best representation for dimsyl sodium? I'm wondering in terms of actual structure, and a "working model". C.f. BuLi's crystal structure versus its working model suitable for our purposes. Also, is dimsyl sodium a worthy article for creation? Or should it just have a mention at DMSO? --Rifleman 82 (talk) 04:10, 1 October 2008 (UTC)
- Oh, I found sodium methylsulfinylmethylide. --Rifleman 82 (talk) 04:54, 1 October 2008 (UTC)
Hoaxes?
Someone pointed me to these two suspicious articles: Erwin Braucheimer and Braucheimer Release Cap. They appear to be hoaxes, but could a few of the other chemists here please take a look? Thanks. -- Ed (Edgar181) 12:52, 2 October 2008 (UTC)
- Obvious hoax, Schnitzel and Braucheimer are not German names (per Google). The image has been taken from http://web.archive.org/web/20040823183819/http://www.emich.edu/public/history/moss/turg1880.jpg and most probably depicts Ivan Turgenev. Cacycle (talk) 13:57, 2 October 2008 (UTC)
- Erwin Schnitzer Braucheimer looks also like a hoax to me as a Swiss. A translation to English could be: “Erwin Escalope Use-Bucket”. :-) --Leyo 21:06, 2 October 2008 (UTC)
- Both have been now been deleted as hoaxes. (Thanks, Rifleman 82!) -- Ed (Edgar181) 14:16, 3 October 2008 (UTC)
Organisation of articles on orbitals / atomic / molecular structure
In Misplaced Pages talk:WikiProject Physics#Organisation of orbital articles I suggested that the Physics project might like to tidy things up, but someone suggested that this project might be more interested — any takers? PJTraill (talk) 17:47, 3 October 2008 (UTC)
PJTraill's concern was about Electron configuration (where he added comments at Talk:Electron configuration#Redundancy with other articles) and Electron cloud (about a merge proposal Talk:Electron cloud#Merger proposal). He also commented on possible redundancy and bad organisation of e.g. Atomic orbital, Electron cloud, Molecular orbital theory, and Molecular orbital. The discussion really should be by chemists primarily. --Bduke (Discussion) 23:24, 3 October 2008 (UTC)
- Note that Atomic orbital and Electron cloud were not tagged for the Chemistry Project, only for the Physics Project. They are now. --Bduke (Discussion) 23:43, 3 October 2008 (UTC)
- Electron configuration is in a pretty sorry state. It does contain some unnescessary material which is redundant to atomic orbital, but more importantly it doesn't contain the sort of coherent explanation which is needed for chemistry! Physchim62 (talk) 00:19, 4 October 2008 (UTC)
IUPAC definition for transition metals
- NOTE: This section is transcluded so the widest-possible number of people can comment
I've been auditing the nav images in element articles to fix wrong neutron counts and giving Lu and Lr the lanthanoid and actinoid coloring, respectively. Part way through, I started to review our definitions for element categories to check them against IUPAC's provisional recommendations. See IUPAC Red Book IR-3.6 GROUPS OF ELEMENTS. Turns out that their specific definition for transition metal deviates from ours in a somewhat embarrassing way:
IUPAC defines transition metals specifically as being those elements in groups 3 to 11. This excludes the group 12 elements!
ED NOTE: Turns out, that IUPAC's approved recommendations define transition metals as either the set of elements in groups 3 to 12 (our current set-up) or the set of elements from 3 to 11 (the set-up in the below table).
Fixing this results in somewhat modified periodic tables (Note, that the expanded 'Other metal' category includes all the post-transition metals plus aluminium):

So, before I finish my audit and fix of the nav images, I'd like to know if I should fix group 12 to be consistent with the provisional IUPAC definition of transition metals. OR should we wait for IUPAC to come out with the final-updated Red Book (comment period ends at the end of 2008)? I'm putting my audit and update of the nav images on hold until we figure this out. --mav (talk) 17:50, 5 October 2008 (UTC)
- I am not sure how many agree to this definition so waiting would be ok. Nergaal (talk) 18:10, 5 October 2008 (UTC)
- I just checked one of my college chemistry textbooks and it agrees with IUPAC. If this definition for transition metals is already widespread, then we may not need to wait for IUPAC's final revision of the Red Book. On the other hand, the updated document may impact other parts of the table and / or nav images. I'm simply not sure how or when we should proceed. --mav (talk) 18:18, 5 October 2008 (UTC)
- Erm, the comment period ended in 2004, according to the root of the file you quoted. The text approved in 2005 was (p. 51):
The elements (except hydrogen) of groups 1, 2 and 13–18 are designated as main group elements and, except in group 18, the first two elements of each main group are termed typical elements. Optionally, the letters s, p, d and f may be used to distinguish different blocks of elements. For example, the elements of groups 3–12 are the d-block elements. These elements are also commonly referred to as the transition elements, though the elements of group 12 are not always included; the f-block elements are sometimes referred to as the inner transition elements.
- As far as I'm aware, there are no new inorganic recommendations planned for four or five years or so (until they get round to sorting out inorganic Preferred IUPAC names). Physchim62 (talk) 18:25, 5 October 2008 (UTC)
- Ah - I saw this and assumed it also applied to the inorganic nomenclature. My bad. I also remember something about unfilled d-suborbitals as part of the definition, which also excludes group 12 elements (with a complication with at least one Hg compound). --mav (talk) 18:35, 5 October 2008 (UTC)
- Definition of this term has always been a problem- whether to base the classification on chemistry or atom electron configuration. I was taught at school (1942 Sherwood Taylor text book) that the transition metals did not include Cu group and Zn group - only then to be told at university that Cu was a transition metal. IMO we should go with current IUPAC - that definition has been around for at least 40 years (Cotton and Wilkinson 2d edition 1966)- it leaves a little problem of colouring in and explaining the position of Zn group which is neither main group nor transition metal, but is in the d block according to our chart- although the chart conflicts with the definition in the article (sic "..highest energy electron is in a d orbital") which would seem to exclude both copper (3d10 4s1) and zinc (3d10 4s2) - if our list of electron configurations is right. Best of luck.--Axiosaurus (talk) 08:32, 6 October 2008 (UTC)
- The current IUPAC definition (quoted above) gives us freedom to include group 12 or not. Let's not forget that Cotton & Wilkinson doesn't class scandium and yttrium as transition metals either, on chemical grounds. Greenwood and Earnshaw agrees with our current classification except for lanthanum and actinium, which they (correctly in my view) class as transition metals. I seem to remember that the edition of Sherwood Taylor that you quote classes thorium and uranium as transition metals and, in the case of thorium ( 7s 5d), a naive or dogmatic application of the electron configuration criterion would force us to do the same! Physchim62 (talk) 08:52, 6 October 2008 (UTC)
- IMHO IUPAC does not clearly define the matter, that's why such a long discussion is needed. My experience is very close to the Axiosaurus' one. The first simple definition refers to empty d orbitals at the elemental state whereas at university I was taught that it's more useful to include group 11 (Cu, Ag, Au) as well because they form ions having empty d orbitals - that is the Cotton Wilkinson definition. This is supported by their behaviour, for instance because they can form coloured complex as the other transition metals. I've never heard that the 12th group (Zn, Cd, Hg) can be included in the transition metals because their behaviour, i.e as catalist, is completely different than the others due to their full d shell. Most of my teachers would have marked as a serious mistake. Cotton Wilkinson (III edition, 1972) includes Scandium and Yttrium between the transition metals. Chemical behaviour should prevail as even Mendeleev based and actually built the periodic table on this characteristic. Some authors try to bridge this describing group 3-12 as d block. Please do not be misled by the shape of the periodic table or, worse, by aestetics issues. Chemistry is an experimental science and sometimes cannot be oversimplyfied. --Avogadro-I (talk) 22:46, 24 November 2008 (UTC)
- The current IUPAC definition (quoted above) gives us freedom to include group 12 or not. Let's not forget that Cotton & Wilkinson doesn't class scandium and yttrium as transition metals either, on chemical grounds. Greenwood and Earnshaw agrees with our current classification except for lanthanum and actinium, which they (correctly in my view) class as transition metals. I seem to remember that the edition of Sherwood Taylor that you quote classes thorium and uranium as transition metals and, in the case of thorium ( 7s 5d), a naive or dogmatic application of the electron configuration criterion would force us to do the same! Physchim62 (talk) 08:52, 6 October 2008 (UTC)
- Definition of this term has always been a problem- whether to base the classification on chemistry or atom electron configuration. I was taught at school (1942 Sherwood Taylor text book) that the transition metals did not include Cu group and Zn group - only then to be told at university that Cu was a transition metal. IMO we should go with current IUPAC - that definition has been around for at least 40 years (Cotton and Wilkinson 2d edition 1966)- it leaves a little problem of colouring in and explaining the position of Zn group which is neither main group nor transition metal, but is in the d block according to our chart- although the chart conflicts with the definition in the article (sic "..highest energy electron is in a d orbital") which would seem to exclude both copper (3d10 4s1) and zinc (3d10 4s2) - if our list of electron configurations is right. Best of luck.--Axiosaurus (talk) 08:32, 6 October 2008 (UTC)
I've always thought that our periodic tables have too many colors and that we could save ourselves a lot of trouble if we got rid of most of them. But I'm afraid I'm in the minority. --Itub (talk) 10:46, 6 October 2008 (UTC)
- But the table is so purty with the colors! And we'd have one less thing to
argue aboutdiscuss - that would be boring. ;) --mav (talk)
Great feedback - thanks for finding the the current recommendations. Looks like IUPAC is giving us some leeway in the definition of transition metals in the approved recommendations. That means that our current table does not conflict with IUPAC. That is all I was worried about. We should therefore leave well-enough alone. We can revisit this if/when IUPAC comes up with a more rigorous definition. But I welcome anybody else to comment just in case we have missed anything. Again - Thank you everybody! --mav (talk) 01:03, 7 October 2008 (UTC)
- Yes, my own opinion is that it's one of those debates that creates more heat than useful work! Physchim62 (talk) 01:21, 7 October 2008 (UTC)
- And don't forget that Misplaced Pages:Naming conventions (chemistry) allows us to go againt IUPAC occasionally, when circumstances demand it! Physchim62 (talk) 01:27, 7 October 2008 (UTC)
It looks like I may be getting in here a little late, but I just wanted to note that in post-transition metal, it claims that the IUPAC definition for transition metals is in conflict with it self. Based upon what I've read here, that doesn't seem to be the case any more. I think it needs to be cleaned up to match the above conclusions. --Wizard191 (talk) 02:04, 8 October 2008 (UTC)
Just a note: first time we get the chance, we should try to get rid of the color differenciation between actinoids and lanthanoids. Nergaal (talk) 17:22, 15 October 2008 (UTC)
- Why and what would replace it? --mav (talk)
- does not add enough information, and within the TMs, the variations in chemistry are larger than those between Ac and Ln's. Any of the two colors used now would be fine, or some random mix of the two too. Nergaal (talk) 15:16, 19 October 2008 (UTC)
- The actinides and lanthanides are distinct enough for us to label them as separate element categories. That combined with the lack of consensus on what is an inner transition tells me that we should leave well enough alone. --mav (talk) 01:06, 20 October 2008 (UTC)
- does not add enough information, and within the TMs, the variations in chemistry are larger than those between Ac and Ln's. Any of the two colors used now would be fine, or some random mix of the two too. Nergaal (talk) 15:16, 19 October 2008 (UTC)
Mercury is considered a transition element under both IUPAC definitions now, because the compound HgF4 has been synthesized in 2007, giving Hg a d electron configuration. Should this be incorporated in the table and the article? Kumorifox (talk) 13:46, 18 May 2009 (UTC)
- Not everyone agrees that mercury is a transition metal due to the observation of HgF4 under exotic conditions. See the article on HgF4 for details. --Itub (talk) 01:12, 19 May 2009 (UTC)
Removing suggestion to Merge d-block and Transition metal
Well I showed up 3 months too late for the fun, but I based on what I read, I am removing the suggestion to merge these two articles. No change in IUPAC recommendations will ever alter Periodic table (by blocks). The blocks must have a number of columns corresponding to the number of electrons that a full subshell can hold. So the d-block must occupy groups 3-12. This is a man-made oversimplification because the chemistry and even the ground state electrons in Periodic_table_(electron_configurations) are messier than the blockiness, but that's ok. Oversimplifications are important because they make reality interesting. "Transition metal" on the other hand, is a convention, not an oversimplification. One bunch of folks call some elements "Transition metals" and another bunch of folks don't, and IUPAC says that's ok. When the most recent IUPAC book says "the elements of group 12 are not always included," they mean not always included in the transition metals. Group 12 has to be in the d-block because if it weren't, then the d-block would only hold 9 columns, meaning 9 electrons maximum in the d-subshell and Kimmie, the cute new 22-year old high-school chemistry teacher, would cry because even the oversimplifications would be too complex to teach, and angry mobs of high school boys who love Kimmie would grab torches and pitchforks and attack IUPAC folks and Misplaced Pages editors for making Kimmie cry. So that's why d-block and Transition metal should not be merged even though IUPAC says they -can- contain the same elements. By the way, Inner transition element and f-block should also be separate articles for the same reason. Conventions and oversimplifications are very, very different. Flying Jazz (talk) 07:47, 18 January 2009 (UTC)
- I agree and I'm glad to see you editing again. :) --mav (talk) 15:40, 19 January 2009 (UTC)
- Well, if Kimmie is wrong, too bad I say. (^_^) Other than that, I agree they should remain separate articles (though that's probably because I am of the firm opinion that the d-block and the transition metals should be different groups). Double sharp (talk) 04:52, 15 September 2013 (UTC)
Electron
I've been working steadily to upgrade this article and I've reached the point where it is discussing more complex interactions such as magnetism, chemistry, electricity and so forth. What I'd like to find out here is how much should this article cover concerning quantum chemistry? My preference is to follow the Misplaced Pages:Summary style guideline and leave the details for other articles. I.e. focus primarily on the electron's role, and less on the chemistry. Do you have any helpful advice on this score? Should I cover, for example, angular momentum coupling in quantum chemistry? The article is already large and I expect it to grow still further. Thank you.—RJH (talk) 16:41, 6 October 2008 (UTC)
P.S. the article's talk page appears to be subject to unhelpful troll baiting, so some caution is recommended there. I'm trying to avoid it.
- Nobody has a problem with it? That's great, thanks.—RJH (talk) 16:02, 9 October 2008 (UTC)
Analytical stubs category
A puzzling thing: A lot of analytical topics (including Analyte, Analytical method, and Applied spectroscopy just to pick a letter...) are filed as physical chemistry stubs. I'm well aware that the line between the two can be a little fuzzy, but the current lists of p-chem stubs and general chem-stubs both include some clear examples to file as analytical stubs. Any objection to marking these out as a separate category? (I see that there used to be one, but it got speedy deleted in 2006 with no reason beyond "upmerged".) - Jaeger5432 | Talk 17:47, 8 October 2008 (UTC)
- I think the main problem is that there are not that many editors active in the area (and so no WikiProject). I have some knowledge of the subject, but I'm a bit snowed under at the moment. Are there any other editors who are willing to take a look at some of these stubs and try to make sure that they're at least not factually inaccurate? Physchim62 (talk) 17:54, 8 October 2008 (UTC)
- If you want to create a new stub type, you have to follow the bureaucratic procedure at Misplaced Pages:STUB#Creating_stub_types (at least if you don't want it be speedy deleted/upmerged again ;-) The main question is, will there be enough stubs of these type? --Itub (talk) 17:56, 8 October 2008 (UTC)
- I think there will be enough. Having the category would help with activity in the area, since it'll provide a more specific focus for work. I'd be happy to spearhead the eventual sorting process, I'm an analytical chemist who'd love to see everything neatly sorted and turned into B class or better... I could go on and estimate the number if that would help the official process. -- Jaeger5432 | Talk 20:07, 8 October 2008 (UTC)
- Yes, if you can come up with an order-of-magnitude estimate of the number, please do so, it will help the official process. Obviously you can set up a workgroup under this project to help coordinate things, if you think that would be useful, but we really need some idea of the scale of the problem as well. My own fear is that the problem is quite large… Physchim62 (talk) 20:34, 8 October 2008 (UTC)
- There were about 30 articles tagged with {{analytical chemistry stub}} and I found and tagged about 30 more that were rated stub in WikiProject Chemistry and WikiProject Spectroscopy. That should be enough to propose creation of the category. --Kkmurray (talk) 03:20, 9 October 2008 (UTC)
- Yes, if you can come up with an order-of-magnitude estimate of the number, please do so, it will help the official process. Obviously you can set up a workgroup under this project to help coordinate things, if you think that would be useful, but we really need some idea of the scale of the problem as well. My own fear is that the problem is quite large… Physchim62 (talk) 20:34, 8 October 2008 (UTC)
- I think there will be enough. Having the category would help with activity in the area, since it'll provide a more specific focus for work. I'd be happy to spearhead the eventual sorting process, I'm an analytical chemist who'd love to see everything neatly sorted and turned into B class or better... I could go on and estimate the number if that would help the official process. -- Jaeger5432 | Talk 20:07, 8 October 2008 (UTC)
- Thanks, Kkmurray. Proposed. It actually qualifies for speedy (re)create, looks like. -- 16:35, 9 October 2008 (UTC)
- If you want to create a new stub type, you have to follow the bureaucratic procedure at Misplaced Pages:STUB#Creating_stub_types (at least if you don't want it be speedy deleted/upmerged again ;-) The main question is, will there be enough stubs of these type? --Itub (talk) 17:56, 8 October 2008 (UTC)
Gallery of Lewis structures in 3-D
What should we do with this article? While this kind of 3D Lewis structure looks reasonable enough, it has a certain "original research" feel to it. --Itub (talk) 17:42, 9 October 2008 (UTC)
- I tagged the poor quality JPG images on Commons. --Leyo 17:53, 9 October 2008 (UTC)
This is definitely OR. Lewis structures might be notable and verifiable but these aren't. If it's going to be anywhere it should be on the author's own website, or (at a push) at Wikibooks.
It's not encyclopaedic content and is therefore unsuitable for Misplaced Pages. Even if we ignore the nonstandard ideas, it's presented in a school textbook style, rather than a concise encyclopaedic style.
Ben (talk) 19:22, 9 October 2008 (UTC)
I agree. This is not encyclopedic and it gives a wrong impression of bond formation. It should be transwikied somewhere, possibly wikibooks. --Bduke (Discussion) 21:05, 9 October 2008 (UTC)
I informed the author, Edguy99. --Leyo 21:52, 9 October 2008 (UTC)
I'm a newbe here so I am not sure of protocol, sorry if I have erred. I am happy to fix the JPG images. With regards to OR, I dont feel that I am doing OR by drawing a 3D view of say a methane molecule that seems to be the natural way one would view a Lewis diagram of Methane if it were visualized in 3D. With regards to writing style, I am happy to work on a more concise encyclopaedic style if given a chance. Edguy99 (talk) 05:03, 10 October 2008 (UTC)
- I appreciate your work that went into your program and into these images. But they do not resemble common molecular visualizations and models and are not generally helpful for readers of Misplaced Pages. Please use the more common rendered VdW radius CPK models or rendered (ball and) stick models for three-dimensional structures or flat circles if you want to to show the electron configurations of simple molecules. The current page should be deleted. Cacycle (talk) 14:17, 10 October 2008 (UTC)
I have grabbed a sample of commonly seen molecule representations for discussion purposes:
My drawing is a representation of this same thing in 3D. It is I think fair to argue what color the dots should be, but there is no doubt there should be 8 dots on carbon and 2 dots on hydrogen I dont feel I am representing anything new, different or original that should disqualify my drawings. In fact, I would argue, that a reasonable person would agree that this is a fair 3D representation of the 2D model, not original research.
My drawing of Carbon and Hydrogen:
I separate and rotate the 3D drawing to give an indication of where the 8 dots for carbon are located in 3D. I do feel this is 'creative' giving me the right to distribute the pictures on such places as Wiki.
Most of the molecules are done with CPK space filling, all I have done is added the Lewis dots to visually represent how they can be connected together to form larger molecules. I drew a couple of molecules smaller and seethrough to help with the visualization. Edguy99 (talk) 17:28, 10 October 2008 (UTC)
- It is those rotating diagrams that I most object to, as they give a quite misleading view of how a bond is broken or formed. --Bduke (Discussion) 22:13, 10 October 2008 (UTC)
- Not to mention that they give the impression that electrons are somehow localized. Other diagrams do this as well, but at least those styles had actually been used before we knew that electrons cannot be localized to this precision. Finally, for your model of carbon and hydrogen, you should realize that carbon has six electrons and hydrogen has one – the diagram is fatally incorrect on this point! Physchim62 (talk) 22:21, 10 October 2008 (UTC)
Lewis diagrams usually only talk about the valence electrons (Carbon has 4, Hydrogen 1). With regards to the rotating diagram, I have animations that do not seperate, they just turn. They look pretty neat too.Edguy99 (talk) 22:45, 10 October 2008 (UTC)
- We dont want this kind of stuff in wikipedia-Chem. 100 years ago, it would be welcome. I dont see many redeeming features aside from the nice colors. --Smokefoot (talk) 22:58, 10 October 2008 (UTC)
- While I appreciate what the author is trying to do and the effort involved, this is a novel representation of molecular bonding, and therefore constitutes original research. Yilloslime (t) 00:17, 11 October 2008 (UTC)
I have read the linked OR document and would like to point out two things:
1/ "to demonstrate that you are not presenting original research, you must cite reliable sources that are directly related to the topic of the article, and that directly support the information as it is presented."
For every 3D image on the page, there are reliable sources that can be cited for its 2D counterpart. This is the same picture, just from a different angle. These pictures whether 2D or 3D are simply a way of illustrating relatively straight forward rules on how oxygen, carbon and nitrogen can be combined. Oxygen has 2 valence holes, nitrogen has 3 and carbon has 4.
2/ "Misplaced Pages editors are encouraged to take photographs or draw pictures or diagrams and upload them, releasing them under the GFDL or another free license, to illustrate articles. This is welcomed because images generally do not propose unpublished ideas or arguments, the core reason behind the NOR policy"
Again, the pictures are only reenforcing the concept behind the rules of how many atoms can combined and in what way.Edguy99 (talk) 01:40, 11 October 2008 (UTC)
- Er, the 3D Lewis images, especially the animated ones, are just plain incorrect. Lewis structures are a fiction (albeit a useful one) that only illustrate a very few things (electron sharing/connectivity is about all) correctly. By taking re-extending the Lewis diagrams into 3D, you're emphasizing the parts of the fiction that are false (especially the spacial arrangement). Adding 3D rotations to a system that is solely about 2D connectivity is not useful at best. The carbon in Image:Carbon1.gif is square-planar! And the individual rotatable atoms (for example, Image:Carbon3d.gif) have the electrons and holes equally spaced in square-bipyramidal, as if each electron and hole is an independent entity, which again is nonsense. The normal planar drawings have electrons paired and correctly illustrate what is sharing and perhaps which atom would be assumed to contribute it and don't go beyond that by extrapolating from the incorrect implications of the model. DMacks (talk) 16:18, 11 October 2008 (UTC)
I have listed the article for deletion, please comment on Misplaced Pages:Articles_for_deletion/Gallery_of_Lewis_structures_in_3-D. Cacycle (talk) 20:16, 11 October 2008 (UTC)
- To be fair, these 3D Lewis structures are somewhat reminiscent of Lewis's cubic atom. However, cubic atoms were published in JACS and are historically notable despite not being used much anymore! --Itub (talk) 06:31, 12 October 2008 (UTC)
I will not be reviving this page in this form but I would like to say that both images (Image:Carbon1.gif and Image:Carbon3d.gif) are the same form. If the large sphere was allowed to rotate longer, I think it would be clearer. I constructed the object starting as tetrahedral object with 4 red dots, then copied, flipped and turned to make 4 white dots equadistance from red dots, but always keeping it as 2 tetrahedrals. I am not sure what this is called and would appreciate being told.Edguy99 (talk) 18:25, 12 October 2008 (UTC)
- The red and white dots together make up the corners of a cube, but if you consider them as two tetrahedra, you have a stella octangula.
- Thank you. I have looked at your pictures and they are very nice. I dont know if you have ever thought about it, but the dots and holes work very well to aide in the construction of 3-d stuff when you are doing it by hand (ie. trying to join one molecule to another in 3-d and figuring out which goes with which). For this shape of molecule, if you give the electrons and holes opposite charges and allow them to be attracted to each other, you can get the hydrocarbon molecules to self-assemble (some assistance required) into correct structures purely based on the relative concentrations of hydrogen and carbon. Same for joining in the Oxygen and Nitrogen molecules to the structure. It saves a lot of work.Edguy99 (talk) 08:53, 13 October 2008 (UTC)
It has come back again. See Misplaced Pages:Articles for deletion/SMemory Animation Software. --Bduke (Discussion) 22:19, 21 October 2008 (UTC)
Perfluorooctanoic acid
Editors might check out perfluorooctanoic acid. This article has grown to 40,000 bytes and 85 specialized references under single authorship over the past few months. The basic storyline is that by virtue of its chemical resilience, perfluorooctanoic acid, mainly a by-product of Teflon production, is pervasive, e.g. it is detectable in most humans, reflecting its easy detectability and stability.--Smokefoot (talk) 14:52, 12 October 2008 (UTC)
- I just noticed that the heavy editor User:Shootbamboo (contribs) has not contributed to any other Misplaced Pages article, making him a clear single-purpose account that started from the very beginning with perfect Misplaced Pages editing skills. It might well be a professional attempt to support court cases using Misplaced Pages. Cacycle (talk) 16:13, 12 October 2008 (UTC)
- The report potentially exploits Misplaced Pages for a hidden agenda. The editor demonstrates no interest in communicating an overview, which is what I thought we do here. Instead the report enumerates articles that discuss occurrence or potential problems/insinuations with PFOA. Shootbamboo is writing a faux-review without enduring the rigor of a real review.--Smokefoot (talk) 17:34, 12 October 2008 (UTC)
- I think "mischief" is a bit a strong, and that the "basic storyline... that by virtue of its chemical resilience, perfluorooctanoic acid, mainly a by-product of Teflon production, is pervasive, e.g. it is detectable in most humans, reflecting its easy detectability and stability," is essentially correct.] The article might give undue weight to the potential health effects of PFOA exposure while giving short shrift to its chemical properties and uses, but as a google news search will show, this is real controversy and something that WP can cover is some depth. Afterall, the Governorator just vetoed a PFOA ban in California, and a global ban on the related PFOS is currently be considered under the Stockholm Convention, so clearly this is hot topic. I'm not saying the article is fine as is, nor am I making assumptions pro- or con- about the WP:SPA involved, I'm only pointing out that this controversy is real, it's probably what most readers who go to the article want to find out about, and it's something the article should discuss at length. Yilloslime (t) 18:34, 12 October 2008 (UTC)
- The report potentially exploits Misplaced Pages for a hidden agenda. The editor demonstrates no interest in communicating an overview, which is what I thought we do here. Instead the report enumerates articles that discuss occurrence or potential problems/insinuations with PFOA. Shootbamboo is writing a faux-review without enduring the rigor of a real review.--Smokefoot (talk) 17:34, 12 October 2008 (UTC)
Other related pages such as DuPont and C-8 and PFOS are probably worth keeping an eye on as well. shoy (reactions) 21:07, 12 October 2008 (UTC)
I had perfluorooctanoic acid on my watchlist before User:Shootbamboo started to work on that article. Overall, I think he (or she) did a good job taking into account the different aspects of that substance. Yes, of course, the environmental issues are much more important here than they might be in most articles on chemical substances. I don't want to go to much into details here, but I have been to three international conferences, where perfluorinated substances like PFOA and PFOS were one of the main topics. I don't share your opinion on the lack of neutrality. PFOA has in fact very good properties and that's described in the article, e.g. “PFOA is an excellent surfactant because perfluorinated surfactants can lower the surface tension of water twice as low as the amount attained with hydrocarbon surfactants.” Of course, the section on the properties might be extended. It might also be a good idea to provide more details on the use of PFOA as an emulsifier in the production of fluoropolymers. IMHO it might also worth providing schemes on this process as well as on the different synthesis routes of PFOA. There are many references, but there are also many in other articles (Polychlorinated dibenzodioxins, Decabromodiphenyl ether). In conclusion, I think the article is quite good, but certainly not perfect and it could be improved by other authors. On the other hand, DuPont and C-8 should be revised. --Leyo 00:46, 14 October 2008 (UTC)
- I don't think this article is that bad either, although it does seem to have too many primary sources. If you want an example of an article that is overly focused on the toxicity, pollution, and suspected carcinogenicity while saying almost nothing about production, properties, etc., try 1,3-dichloropropene. --Itub (talk) 05:57, 14 October 2008 (UTC)
This is almost certainly from the "Environmental Working Group" (EWG, see also). I must say that it's better than the utter c*** they have produced in the past: however, it is still trying to prove a point, rather than discussing issues from a neutral point of view. Cleanup needed, IMHO. Physchim62 (talk) 00:07, 15 October 2008 (UTC)
References in Misplaced Pages articles - guidelines for how many
The previous section describes a debate about an article on perfluorooctanoic acid, which some of us think contains too many references and in a way constitutes original research. So now may be a good time to broach policy issues raised by this debate: how many references are appropriate for an article about a chemical compound, recognizing that some variability is inevitable? Experienced editors have noticed that for many chemical compounds, Googling locates lots of reports on toxicology and hazards. Even setting aside the tox/environmental papers generated through Googling, one could fill many articles with thousands of references of varying detail. Using Chemical Abstracts, I checked two semi-random compounds (mid-significance, with well-known, mid-level hazards): nitrobenzene gives 12,000 hits and methyl bromide generates 6000 hits. Obviously we dont want that many references. So, IMHO, the Wiki-Chem community need to discuss guidelines for referencing in our style guide.--Smokefoot (talk) 22:32, 14 October 2008 (UTC)
- I would say that Misplaced Pages is a tertiary source, and so its references should normally be secondary sources (ie, peer-reviewed review articles or generally-accepted textbooks). If no secondary sources are available, that starts to raise questions about the articles notability, at least as an independent article and not as a section in another article. In terms of chemical safety, this is particularly true: if nobody else has felt it necessary to do make a toxicological review then it shouldn't be for WP to say that X and Y's study is particularly important (because, quite simply, it probably isn't). See also WP:SOAPBOX and Misplaced Pages:Requests for arbitration/Depleted uranium. Physchim62 (talk) 23:53, 14 October 2008 (UTC)
- I support using secondary sources as references generally. However, I don't see a reason for not using primary sources (e.g. peer-reviewed journals, company reports) to include new findings. Of course, these findings should not weighted by Misplaced Pages authors. I agree that 85 references are a lot, but I don't think a fixed limit of references is a good idea.
- Why not discussing guidelines on the need of citing references for chemical properties in the chemobox. Presently, hardly any chemobox is sufficiently referenced. In de-WP, the vast majority of the (non-trivial) properties in the chemoboxes are referenced now, after having put efforts on that during the last few month. When we added missing references, we discovered quite a lot of incorrect values. This would most probably also be true for en-WP (e.g. this old version concerning the density). --Leyo 00:41, 15 October 2008 (UTC)
- I don't think there's an easy answer to "how many references?". Some chemicals have many uses, some don't. Some are the center of a controversy (would you like some melamine with that milk?), some at the center of several controversies (think DDT), and some are not controversial at all (pentane comes to mind). Some are historically interesting (e.g. Tartaric acid), some are just another derivative (e.g. Butylamine.) I do agree that we should try to draw from secondary and tertiary sources, rather than primary ones, and that concept applies to toxicology as well as chemical properties, uses, history, etc. We should not, however, scrupulously exclude primary sources, especially those that have come out since the last review and/or have garnered media attention. Historically important primary sources (i.e., ...first synthesized in 1865 by some german guy), and illustrative examples can also be appropriate. Also, the introductions of primary sources are sometimes mini-reviews of a topic intended to put the authors' new work in perspective, and we should be able to cite these the same way we might cite a review. For example, a research article about a chiral, cationic, tridentate ligand might started off by saying, "Chiral, cationic, tridentate ligands have received a lot of attention in the last few years as potential ancillary ligands for unlikley catalytic reaction XYZ" and then cite several examples, before going on to describe the authors' work on a new ligand of that type. Whether the work described in the research article is notable or not is one thing, but we should be allowed to cite the paper as evidence that this general type ligand is being investigated for XYZ. Yilloslime (t) 00:44, 15 October 2008 (UTC)
- Firstly I agree with Yilloslime above regarding the the mini review at the beginning of many primary source articles.
- Regarding the use of primary sources - in the area of inorganics I find that many chemical compound articles flagged as stubs require references to primary sources to improve them siginificantly as secondary sources e.g. major text books, review articles have insufficient compound specific detail.
- Regarding the number of references - I believe that this is best taken on a case by case basis and I would avoid being too prescriptive. The driver for the number of references should be the content of the article and the level of detail - if we get that right and reference accordingly- secondary sources for preference and primary where necessary then the reference count for that article is, as Goldilocks would say, just right.--Axiosaurus (talk) 16:55, 19 October 2008 (UTC)
Please expand Half cell
There was an expansion request at Half cell which was redundant with its stub note, so I am moving the request here. I've been trying to overhaul the Battery (electricity) and Galvanic cell-related articles. Orange Knight of Passion (talk) 20:50, 16 October 2008 (UTC)
Symbol for Chemical Equilibrium
Could someone from this Wikiproject weigh in at Wikipedia_talk:Accessibility#Equilibrium_symbol regarding various ways of rendering the symbol for chemical equilibrium (⇌)? Thanks- ~ L'Aquatique 05:06, 18 October 2008 (UTC)
List of elements
Hi, two of the periodic table related FLs have merge tags (List of elements by name and List of elements by symbol) and I found this discussion, where there appears to be consensus for a merge, but nobody has done it for whatever reason. I was going to remove the tags, but first I thought I would see if there was still any interest in a merge.
If you want to merge the pages, that would be fine, but the FLs would have to be formally delisted first (which shouldn't be a problem, it has been done before). Is there someone within this project who would be willing to do this? I would, but I don't have a lot of expertise with the periodic table so it would probably be better for someone familiar with the subject to do it. If nobody responds, then I am just going to remove the tags. -- Scorpion 03:42, 21 October 2008 (UTC)
The featured lists List of elements by symbol and List of elements by name been nominated for removal. You can comment here and here. -- Scorpion 22:56, 21 October 2008 (UTC)
Animation
I found this particularly useful in explaining the basics behind free-radical polymerisation and a free version for wikipedia would be a very useful addition to the relevant article if anyone has the time and knowhow. Noodle snacks (talk) 09:27, 26 October 2008 (UTC)
Cold fusion
HELP! We have Cold Fusion proponents dramatically asserting ownership over cold fusion. I need all the help I can get. ScienceApologist (talk) 15:57, 27 October 2008 (UTC)
- nnnn

