| Revision as of 17:33, 22 May 2010 view source24.254.227.107 (talk) spelling error fixed← Previous edit | Revision as of 22:49, 22 May 2010 view source William M. Connolley (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers66,008 edits Undid revision 363582686 by 24.254.227.107 (talk) wasn't an errorNext edit → | ||
| Line 60: | Line 60: | ||
| Carbon dioxide has no liquid state at pressures below 5.1 ]. At 1 atmosphere (near mean sea level pressure), the gas ] directly to a solid at temperatures below {{convert|-78|C|F K}} and the solid ] directly to a gas above −78 °C. In its solid state, carbon dioxide is commonly called ]. | Carbon dioxide has no liquid state at pressures below 5.1 ]. At 1 atmosphere (near mean sea level pressure), the gas ] directly to a solid at temperatures below {{convert|-78|C|F K}} and the solid ] directly to a gas above −78 °C. In its solid state, carbon dioxide is commonly called ]. | ||
| CO<sub>2</sub> is an ]: an aqueous solution turns ] from blue to pink. It is the ] of ], an acid which is unstable in aqueous solution, from which it cannot be concentrated. However it can be produced by irradiating frozen mixtures of water and carbon dioxide in |
CO<sub>2</sub> is an ]: an aqueous solution turns ] from blue to pink. It is the ] of ], an acid which is unstable in aqueous solution, from which it cannot be concentrated. However it can be produced by irradiating frozen mixtures of water and carbon dioxide in vacuum. In organisms carbonic acid production is catalysed by the ], ]. | ||
| : {{chem|CO|2}} + {{chem|H|2|O}} ⇌ {{chem|H|2|CO|3}} | : {{chem|CO|2}} + {{chem|H|2|O}} ⇌ {{chem|H|2|CO|3}} | ||
Revision as of 22:49, 22 May 2010
"CO2" redirects here. For the UK postal district, see CO postcode area.
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Carbon dioxide | |||
| Other names Carbonic acid gas; carbonic anhydride; dry ice (solid) | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.271 | ||
| EC Number |
| ||
| E number | E290 (preservatives) | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 1013 Solid (dry ice): 1845 Mixtures with Ethylene oxide: 1952, 3300 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | CO2 | ||
| Molar mass | 44.010 g/mol | ||
| Appearance | colorless, odorless gas | ||
| Density | 1.562 g/mL (solid at 1 atm and −78.5 °C) 0.770 g/mL (liquid at 56 atm and 20 °C) 1.977 g/L (gas at 1 atm and 0 °C) 849.6 g/L (supercritical fluid at 150 atm and 30 °C | ||
| Melting point | −78.5 °C; −109.2 °F; 194.7 K | ||
| Boiling point | −56.6 °C; −69.8 °F; 216.6 K | ||
| Solubility in water | 1.45 g/L at 25 °C, 100 kPa | ||
| Acidity (pKa) | 6.35, 10.33 | ||
| Refractive index (nD) | 1.1120 | ||
| Viscosity | 0.07 cP at −78 °C | ||
| Dipole moment | zero | ||
| Structure | |||
| Molecular shape | linear | ||
| Related compounds | |||
| Other anions | Carbon disulfide Carbon diselenide | ||
| Other cations | Silicon dioxide Germanium dioxide Tin dioxide Lead dioxide | ||
| Supplementary data page | |||
| Carbon dioxide (data page) | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Carbon dioxide (chemical formula CO2) is a chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom. It is a gas at standard temperature and pressure and exists in Earth's atmosphere in this state. CO2 is a trace gas comprising 0.039% of the atmosphere.
Carbon dioxide is used by plants during photosynthesis to make sugars, which may either be consumed in respiration or used as the raw material to produce other organic compounds needed for plant growth and development. It is produced during respiration by plants, and by all animals, fungi and microorganisms that depend either directly or indirectly on plants for food. It is thus a major component of the carbon cycle. Carbon dioxide is generated as a by-product of the combustion of fossil fuels or the burning of vegetable matter, among other chemical processes. Amounts of carbon dioxide are emitted from volcanoes and other geothermal processes such as hot springs and geysers and by the dissolution of carbonates in crustal rocks.
As of April 2010, carbon dioxide in the Earth's atmosphere is at a concentration of 391 ppm by volume. Atmospheric concentrations of carbon dioxide fluctuate slightly with the change of the seasons, driven primarily by seasonal plant growth in the Northern Hemisphere. Concentrations of carbon dioxide fall during the northern spring and summer as plants consume the gas, and rise during the northern autumn and winter as plants go dormant, die and decay. Taking all this into account, the concentration of CO2 grew by about 2 ppm in 2009. Carbon dioxide is a greenhouse gas as it transmits visible light but absorbs strongly in the infrared and near-infrared.
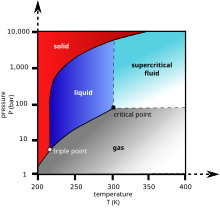
Carbon dioxide has no liquid state at pressures below 5.1 atmospheres. At 1 atmosphere (near mean sea level pressure), the gas deposits directly to a solid at temperatures below −78 °C (−108 °F; 195 K) and the solid sublimes directly to a gas above −78 °C. In its solid state, carbon dioxide is commonly called dry ice.
CO2 is an acidic oxide: an aqueous solution turns litmus from blue to pink. It is the anhydride of carbonic acid, an acid which is unstable in aqueous solution, from which it cannot be concentrated. However it can be produced by irradiating frozen mixtures of water and carbon dioxide in vacuum. In organisms carbonic acid production is catalysed by the enzyme, carbonic anhydrase.
- CO
2 + H
2O ⇌ H
2CO
3
CO2 is toxic in higher concentrations: 1% (10,000 ppm) will make some people feel drowsy. Concentrations of 7% to 10% cause dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.
Chemical and physical properties

Carbon dioxide is colorless. At low concentrations, the gas is odorless. At higher concentrations it has a sharp, acidic odor. It will act as an asphyxiant and an irritant. When inhaled at concentrations much higher than usual atmospheric levels, it can produce a sour taste in the mouth and a stinging sensation in the nose and throat. These effects result from the gas dissolving in the mucous membranes and saliva, forming a weak solution of carbonic acid. This sensation can also occur during an attempt to stifle a burp after drinking a carbonated beverage. Amounts above 5,000 ppm are considered very unhealthy, and those above about 50,000 ppm (equal to 5% by volume) are considered dangerous to animal life.
At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m, about 1.5 times that of air. The carbon dioxide molecule (O=C=O) contains two double bonds and has a linear shape. It has no electrical dipole, and as it is fully oxidized, it is moderately reactive and is non-flammable, but will support the combustion of metals such as magnesium.
Above -78.51° C or -109.3° F, carbon dioxide changes directly from a solid phase to a gaseous phase through sublimation, or from gaseous to solid through deposition. Solid carbon dioxide is normally called "dry ice", a generic trademark. It was first observed in 1825 by the French chemist Charles Thilorier. Dry ice is commonly used as a cooling agent, and it is relatively inexpensive. A convenient property for this purpose is that solid carbon dioxide sublimes directly into the gas phase leaving no liquid. It can often be found in grocery stores and laboratories, and it is also used in the shipping industry. The largest non-cooling use for dry ice is blast cleaning.
Liquid carbon dioxide forms only at pressures above 5.1 atm; the triple point of carbon dioxide is about 518 kPa at -56.6 °C (See phase diagram, above). The critical point is 7.38 MPa at 31.1 °C.
An alternative form of solid carbon dioxide, an amorphous glass-like form, is possible, although not at atmospheric pressure. This form of glass, called carbonia, was produced by supercooling heated CO2 at extreme pressure (40–48 GPa or about 400,000 atmospheres) in a diamond anvil. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon (silica glass) and germanium. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts back to gas when pressure is released.
See also: Supercritical carbon dioxide, dry ice, and MO diagramHistory of human understanding
Carbon dioxide was one of the first gases to be described as a substance distinct from air. In the seventeenth century, the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" or "wild spirit" (spiritus sylvestre).
The properties of carbon dioxide were studied more thoroughly in the 1750s by the Scottish physician Joseph Black. He found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called "fixed air." He observed that the fixed air was denser than air and supported neither flame nor animal life. Black also found that when bubbled through an aqueous solution of lime (calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley published a paper entitled Impregnating Water with Fixed Air in which he described a process of dripping sulfuric acid (or oil of vitriol as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas. This was the invention of Soda water.
Carbon dioxide was first liquefied (at elevated pressures) in 1823 by Humphry Davy and Michael Faraday. The earliest description of solid carbon dioxide was given by Charles Thilorier, who in 1834 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid CO2.
Isolation and production
Carbon dioxide may be obtained from air distillation. However, this yields only very small quantities of CO2. A large variety of chemical reactions yield carbon dioxide, such as the reaction between most acids and most metal carbonates. For example, the reaction between hydrochloric acid and calcium carbonate (limestone or chalk) is depicted below:
- 2 HCl+ CaCO
3→ CaCl
2+ H
2CO
3
The H
2CO
3 then decomposes to water and CO2. Such reactions are accompanied by foaming or bubbling, or both. In industry such reactions are widespread because they can be used to neutralize waste acid streams.
The production of quicklime (CaO) a chemical that has widespread use, from limestone by heating at about 850 °C also produces CO2:
- CaCO
3→ CaO + CO
2
The combustion of all carbon containing fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), but also of coal and wood, will yield carbon dioxide and, in most cases, water. As an example the chemical reaction between methane and oxygen is given below.
- CH
4+ 2 O
2→ CO
2+ 2 H
2O
Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:
- Fe
2O
3+ 3 CO → 2 Fe + 3 CO
2
Yeast metabolizes sugar to produce carbon dioxide and ethanol, also known as alcohol, in the production of wines, beers and other spirits, but also in the production of bioethanol:
- C
6H
12O
6 → 2 CO
2+ 2 C
2H
5OH
All aerobic organisms produce CO
2 when they oxidize carbohydrates, fatty acids, and proteins in the mitochondria of cells. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration, anaerobic respiration and photosynthesis). Photoautotrophs (i.e. plants, cyanobacteria) use another modus operandi: Plants absorb CO
2 from the air, and, together with water, react it to form carbohydrates:
- nCO2 + nH
2O → (CH
2O)n + nO
2
Carbon dioxide is soluble in water, in which it spontaneously interconverts between CO2 and H
2CO
3 (carbonic acid). The relative concentrations of CO
2, H
2CO
3, and the deprotonated forms HCO
3 (bicarbonate) and CO
3(carbonate) depend on the pH. In neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater, while in very alkaline water (pH > 10.4) the predominant (>50%) form is carbonate. The bicarbonate and carbonate forms are very soluble, such that air-equilibrated ocean water (mildly alkaline with typical pH = 8.2 – 8.5) contains about 120 mg of bicarbonate per liter.
Industrial production
Industrial carbon dioxide is produced mainly from six processes:
- From combustion of fossil fuels and wood;
- As a by-product of hydrogen production plants, where methane is converted to CO2;
- As a by-product of fermentation of sugar in the brewing of beer, whisky and other alcoholic beverages;
- From thermal decomposition of limestone, CaCO
3, in the manufacture of lime, CaO; - As a by-product of sodium phosphate manufacture;
- Directly from natural carbon dioxide springs, where it is produced by the action of acidified water on limestone or dolomite.
Uses

Carbon dioxide is used by the food industry, the oil industry, and the chemical industry. It is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately 60 bar (870 psi, 59 atm), allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminum capsules of CO2 are also sold as supplies of compressed gas for airguns, paintball markers, inflating bicycle tires, and for making carbonated water. Rapid vaporization of liquid carbon dioxide is used for blasting in coal mines. High concentrations of carbon dioxide can also be used to kill pests, such as the Common Clothes Moth.
Drinks
Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation in beer and sparkling wine came about through natural fermentation, but many manufacturers carbonate these drinks artificially. In the case of bottled and kegged beer, artificial carbonation is now the most common method used. With the exception of British Real Ale, draught (draft) beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurised carbon dioxide, often mixed with nitrogen.
Foods
A candy called Pop Rocks is pressurized with carbon dioxide gas at about 40 bar (600 psi). When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents produce carbon dioxide to cause dough to rise. Baker's yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acids.
Pneumatic systems
Carbon dioxide is one of the most commonly used compressed gases for pneumatic (pressurized gas) systems in portable pressure tools and combat robots.
Fire extinguisher
Carbon dioxide extinguishes flames, and some fire extinguishers, especially those designed for electrical fires, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, as it is so dry. Carbon dioxide has also been widely used as an extinguishing agent in fixed fire protection systems for local application of specific hazards and total flooding of a protected space, (National Fire Protection Association Code 12). International Maritime Organization standards also recognize carbon dioxide systems for fire protection of ship holds and engine rooms. Carbon dioxide based fire protection systems have been linked to several deaths, due to the fact that it does not support life in the concentrations used to extinguish fire (40% or so), however, it is not considered to be toxic to humans. A review of CO2 systems (Carbon Dioxide as a Fire Suppressant: Examining the Risks, US EPA) identified 51 incidents between 1975 and the date of the report, causing 72 deaths and 145 injuries.
Welding
Carbon dioxide also finds use as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle than those made in more inert atmospheres, and that such weld joints deteriorate over time because of the formation of carbonic acid. It is used as a welding gas primarily because it is much less expensive than more inert gases such as argon or helium.
Caffeine removal
Liquid carbon dioxide is a good solvent for many lipophilic organic compounds, and is used to remove caffeine from coffee. First, the green coffee beans are soaked in water. The beans are placed in the top of a column seventy feet (21 m) high. Then supercritical carbon dioxide in fluid form at about 93 °C enters at the bottom of the column. The caffeine diffuses out of the beans and into the carbon dioxide.
Pharmaceutical and other chemical processing
Carbon dioxide has begun to attract attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It's used by some dry cleaners for this reason. (See green chemistry.)
In the chemical industry, carbon dioxide is used for the production of urea, carbonates and bicarbonates, and sodium salicylate.
Agricultural and biological applications
Plants require carbon dioxide to conduct photosynthesis. Greenhouses may (and of large size - must) enrich their atmospheres with additional CO2 to sustain plant life and growth. A photosynthesis-related drop (by a factor less than two) in carbon dioxide concentration in a greenhouse compartment would kill green plants, or, at least, completely stop their growth. At very high concentrations (a factor of 100 or more higher than its atmospheric concentration), carbon dioxide can be toxic to animal life, so raising the concentration to 10,000 ppm (1%) or higher for several hours will eliminate pests such as whiteflies and spider mites in a greenhouse.
It has been proposed that carbon dioxide from power generation be bubbled into ponds to grow algae that could then be converted into biodiesel fuel. Carbon dioxide is already increasingly used in greenhouses as the main carbon source for Spirulina algae.
In medicine, up to 5% carbon dioxide (130 times the atmospheric concentration) is added to pure oxygen for stimulation of breathing after apnea and to stabilize the O
2/CO
2 balance in blood.
Lasers

A common type of industrial gas laser is the carbon dioxide laser.
Polymers and plastics
Carbon dioxide can also be combined with limonene oxide from orange peels or other epoxides to create polymers and plastics.
Oil recovery
Carbon dioxide is used in enhanced oil recovery where it is injected into or adjacent to producing oil wells, usually under supercritical conditions. It acts as both a pressurizing agent and, when dissolved into the underground crude oil, significantly reduces its viscosity, enabling the oil to flow more rapidly through the earth to the removal well. In mature oil fields, extensive pipe networks are used to carry the carbon dioxide to the injection points.
As refrigerants
Liquid and solid carbon dioxide are important refrigerants, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called "dry ice" and is used for small shipments where refrigeration equipment is not practical.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the discovery of R-12 and is likely to enjoy a renaissance due to environmental concerns. Its physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to its operation at pressures of up to 130 bar (1880 psi), CO2 systems require highly resistant components that have already been developed for mass production in many sectors. In automobile air conditioning, in more than 90% of all driving conditions for latitudes higher than 50°, R744 operates more efficiently than systems using R-134a. Its environmental advantages (GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, hot water heat pumps, among others. Coca-Cola has fielded CO2-based beverage coolers and the U.S. Army is interested in CO2 refrigeration and heating technology.
By the end of 2007, the global automobile industry is expected to decide on the next-generation refrigerant in car air conditioning. CO2 is one discussed option.(see The Cool War)
Coal bed methane recovery
In enhanced coal bed methane recovery, carbon dioxide is pumped into the coal seam to displace methane.
Wine making
Carbon dioxide in the form of dry ice is often used in the wine making process to cool down bunches of grapes quickly after picking to help prevent spontaneous fermentation by wild yeasts. The main advantage of using dry ice over regular water ice is that it cools the grapes without adding any additional water that may decrease the sugar concentration in the grape must, and therefore also decrease the alcohol concentration in the finished wine.
Dry ice is also used during the cold soak phase of the wine making process to keep grapes cool. The carbon dioxide gas that results from the sublimation of the dry ice tends to settle to the bottom of tanks because it is heavier than regular air. The settled carbon dioxide gas creates an hypoxic environment which helps to prevent bacteria from growing on the grapes until it is time to start the fermentation with the desired strain of yeast.
Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, the process used to produce Beaujolais wine.
Carbon dioxide is sometimes used to top up wine bottles or other storage vessels such as barrels to prevent oxidation, though it has the problem that it can dissolve into the wine, making a previously still wine slightly fizzy. For this reason, other gasses such as nitrogen or argon are preferred for this process by professional wine makers.
pH control
Carbon dioxide can be used as a mean of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH level from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids.
CO2 is also used in the keeping of reef aquaria, where it is commonly used in calcium reactors to temporarily lower the pH of water being passed over calcium carbonate in order to allow the calcium carbonate to dissolve into the water more freely where it is used by some corals to build their skeleton.
In the Earth's atmosphere
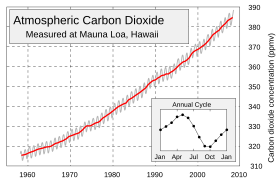
Carbon dioxide in earth's atmosphere is considered a trace gas currently occurring at an average concentration of about 383 parts per million by volume or 582 parts per million by mass. The total mass of atmospheric carbon dioxide is 3.0×10 kg (3,000 gigatonnes). Its concentration varies seasonally (see graph at right) and also considerably on a regional basis, especially near the ground. In urban areas concentrations are generally higher and indoors they can reach 10 times background levels. Carbon dioxide is a greenhouse gas.

Five hundred million years ago carbon dioxide was 20 times more prevalent than today, decreasing to 4-5 times during the Jurassic period and then slowly declining with a particularly swift reduction occurring 49 million years ago. Human activities such as the combustion of fossil fuels and deforestation have caused the atmospheric concentration of carbon dioxide to increase by about 35% since the beginning of the age of industrialization.
Up to 40% of the gas emitted by some volcanoes during subaerial eruptions is carbon dioxide. It is estimated that volcanoes release about 130-230 million tonnes (145-255 million tons) of CO2 into the atmosphere each year. This is about 100 times smaller than the sources from human activity. Carbon dioxide is also produced by hot springs such as those at the Bossoleto site near Rapolano Terme in Tuscany, Italy. Here, in a bowl-shaped depression of about 100 m diameter, local concentrations of CO2 rise to above 75% overnight, sufficient to kill insects and small animals, but it warms rapidly when sunlit and the gas is dispersed by convection during the day. Locally high concentrations of CO2, produced by disturbance of deep lake water saturated with CO2 are thought to have caused 37 fatalities at Lake Monoun, Cameroon in 1984 and 1700 casualties at Lake Nyos, Cameroon in 1986. Emissions of CO2 by human activities are currently more than 130 times greater than the quantity emitted by volcanoes, amounting to about 27 billion tonnes per year.
In the oceans
Main article: Carbon cycleThere is about fifty times as much carbon dissolved in the sea water of the oceans in the form of CO2 and carbonic acid, bicarbonate and carbonate ions as exists in the atmosphere. The oceans act as an enormous carbon sink, and have taken up about a third of CO2 emitted by human activity. Gas solubility decreases as the temperature of water increases (except when both pressure exceeds 300 bar and temperature exceeds 393 K, only found near deep geothermal vents) and therefore the rate of uptake from the atmosphere decreases as ocean temperatures rise.
Most of the CO2 taken up by the ocean forms carbonic acid in equilibrium with bicarbonate and carbonate ions. Some is consumed in photosynthesis by organisms in the water, and a small proportion of that sinks and leaves the carbon cycle. Increased CO2 in the atmosphere has led to decreasing alkalinity of seawater and there is concern that this may adversely affect organisms living in the water. In particular, with decreasing alkalinity, the availability of carbonates for forming shells decreases, although there's evidence of increased shell production by certain species under increased CO2 content.
NOAA states in their May 2008 "State of the science fact sheet for ocean acidification" that:
"The oceans have absorbed about 50% of the carbon dioxide (CO2) released from the burning of fossil fuels, resulting in chemical reactions that lower ocean pH. This has caused an increase in hydrogen ion (acidity) of about 30% since the start of the industrial age through a process known as “ocean acidification.” A growing number of studies have demonstrated adverse impacts on marine organisms, including:
- The rate at which reef-building corals produce their skeletons decreases.
- The ability of marine algae and free-swimming zooplankton to maintain protective shells is reduced.
- The survival of larval marine species, including commercial fish and shellfish, is reduced."
Also, the Intergovernmental Panel on Climate Change (IPCC) writes in their Climate Change 2007: Synthesis Report :
"The uptake of anthropogenic carbon since 1750 has led to the ocean becoming more acidic with an average decrease in pH of 0.1 units. Increasing atmospheric CO2 concentrations lead to further acidification .. While the effects of observed ocean acidification on the marine biosphere are as yet undocumented, the progressive acidification of oceans is expected to have negative impacts on marine shell-forming organisms (e.g. corals) and their dependent species."
IPCC also includes in its last report that with a probability greater than 0.66: "the resilience of many ecosystems is likely to be exceeded in this century by an unprecedented combination of climate change, associated disturbances (e.g. flooding, drought, wildfire, insects, ocean acidification) and other global change drivers (e.g. landuse change, pollution, fragmentation of natural systems, overexploitation of resources)."
Some marine calcifying organisms (like coral reefs) have been singled out by major research agencies, including NOAA, OSPAR commission, NANOOS and the IPCC, because their most current research shows that ocean acidification should be expected to impact them negatively.
Biological role
Carbon dioxide is an end product in organisms that obtain energy from breaking down sugars, fats and amino acids with oxygen as part of their metabolism, in a process known as cellular respiration. This includes all plants, animals, many fungi and some bacteria. In higher animals, the carbon dioxide travels in the blood from the body's tissues to the lungs where it is exhaled. In plants using photosynthesis, carbon dioxide is absorbed from the atmosphere.
Role in photosynthesis
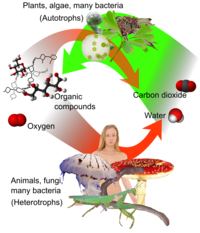
Plants remove carbon dioxide from the atmosphere by photosynthesis, also called carbon assimilation, which uses light energy to produce organic compounds (cellulose, lipids, and various proteins) by combining carbon dioxide and water. Free oxygen is released as gas from the decomposition of water molecules, while the hydrogen is split into its protons and electrons and used to generate chemical energy via photophosphorylation. This energy is required for the fixation of carbon dioxide in the Calvin cycle to make 3-phosphoglycerate that is used in metabolism, to construct sugars that can be used as an energy source within the plant through respiration and as the raw material for the construction of more complex organic molecules, such as polysaccharides, nucleic acids and proteins during growth.
Plants can grow up to 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Some people (for example David Bellamy) believe that as the concentration of CO2 rises in the atmosphere that it will lead to faster plant growth and therefore increase food production. Such views are controversial. Studies have shown that increased CO2 leads to fewer stomata developing on plants which leads to reduced water usage. Studies using FACE have shown that increases in CO2 lead to decreased concentration of micronutrients in crop plants. This may have knock-on effects on other parts of ecosystems as herbivores will need to eat more food to gain the same amount of protein.
Plants also emit CO2 during respiration, and so the majority of plants and algae, which use C3 photosynthesis, are only net absorbers during the day. Though a growing forest will absorb many tons of CO2 each year, the World Bank writes that a mature forest will produce as much CO2 from respiration and decomposition of dead specimens (e.g. fallen branches) as is used in biosynthesis in growing plants. However six experts in biochemistry, biogeology, forestry and related areas writing in the science journal Nature that "Our results demonstrate that old-growth forests can continue to accumulate carbon, contrary to the long-standing view that they are carbon neutral." Mature forests are valuable carbon sinks, helping maintain balance in the Earth's atmosphere. Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved CO2 in the upper ocean and thereby promotes the absorption of CO2 from the atmosphere.
Toxicity
See also: Carbon dioxide poisoning
Carbon dioxide content in fresh air (averaged between sea-level and 10 hPa level, i.e. about 30 km altitude) varies between 0.036% (360 ppm) and 0.039% (390 ppm), depending on the location.
Prolonged exposure to moderate concentrations can cause acidosis and adverse effects on calcium phosphorus metabolism resulting in increased calcium deposits in soft tissue. Carbon dioxide is toxic to the heart and causes diminished contractile force.
Toxicity and its effects increase with the concentration of CO2, here given in volume percent of CO2 in the air:
- 1% can cause drowsiness with prolonged exposure.
- At 2% it is mildly narcotic and causes increased blood pressure and pulse rate, and causes reduced hearing.
- At about 5% it causes stimulation of the respiratory centre, dizziness, confusion and difficulty in breathing accompanied by headache and shortness of breath.. Panic attacks may also occur at this concentration.
- At about 8% it causes headache, sweating, dim vision, tremor and loss of consciousness after exposure for between five and ten minutes.
Due to the health risks associated with carbon dioxide exposure, the U.S. Occupational Safety and Health Administration says that average exposure for healthy adults during an eight-hour work day should not exceed 5,000 ppm (0.5%). The maximum safe level for infants, children, the elderly and individuals with cardio-pulmonary health issues is significantly less. For short-term (under ten minutes) exposure, the U.S. National Institute for Occupational Safety and Health (NIOSH) and American Conference of Government Industrial Hygienists (ACGIH) limit is 30,000 ppm (3%). NIOSH also states that carbon dioxide concentrations exceeding 4% are immediately dangerous to life and health although physiological experiments show that such levels can be tolerated for some time .
Adaptation to increased levels of CO2 occurs in humans. Continuous inhalation of CO2 can be tolerated at three percent inspired concentrations for at least one month and four percent inspired concentrations for over a week. It was suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a submarine) since the adaptation is physiological and reversible. Decrement in performance or in normal physical activity does not happen at this level. However, it should be noted that submarines have carbon dioxide scrubbers which reduce a significant amount of the CO2 present.
These figures are valid for pure carbon dioxide. In indoor spaces occupied by people the carbon dioxide concentration will reach higher levels than in pure outdoor air. Concentrations higher than 1,000 ppm will cause discomfort in more than 20% of occupants, and the discomfort will increase with increasing CO2 concentration. The discomfort will be caused by various gases coming from human respiration and perspiration, and not by CO2 itself. At 2,000 ppm the majority of occupants will feel a significant degree of discomfort, and many will develop nausea and headaches. The CO2 concentration between 300 and 2,500 ppm is used as an indicator of indoor air quality.
Acute carbon dioxide toxicity is sometimes known by the names given to it by miners: blackdamp (also called choke damp or stythe). Blackdamp is primarily nitrogen and carbon dioxide and kills via suffocation (having displaced oxygen). Miners would try to alert themselves to dangerous levels of blackdamp and other gasses in a mine shaft by bringing a caged canary with them as they worked. The canary is more sensitive to environmental gasses than humans and as it became unconscious would stop singing and fall off its perch. The Davey lamp could also detect high levels of blackdamp (which collect near the floor) by burning less brightly, while methane, another suffocating gas and explosion risk would make the lamp burn more brightly).
Carbon dioxide differential above outdoor levels at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person. CO2 is considered to be a surrogate for human bio-effluents and may correlate with other indoor pollutants. Higher CO2 concentrations are associated with occupant health, comfort and performance degradation. ASHRAE Standard 62.1-2007 ventilation rates may result in indoor levels up to 2,100 ppm above ambient outdoor conditions. Thus if the outdoor ambient is 400 ppm, indoor levels may reach 2,500 ppm with ventilation rates that meet this industry consensus standard. Levels in poorly ventilated spaces can be found even higher than this (range of 3,000 or 4,000).
Human physiology
See also: Arterial blood gasCO2 is carried in blood in three different ways. (The exact percentages vary depending whether it is arterial or venous blood).
- Most of it (about 70% – 80%) is converted to bicarbonate ions HCO
3 by the enzyme carbonic anhydrase in the red blood cells, by the reaction CO2 + H2O → H2CO3 → H + HCO
3. - 5% – 10% is dissolved in the plasma
- 5% – 10% is bound to hemoglobin as carbamino compounds
Hemoglobin, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. The decreased binding to carbon dioxide in the blood due to increased oxygen levels is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr Effect.
Carbon dioxide is one of the mediators of local autoregulation of blood supply. If its levels are high, the capillaries expand to allow a greater blood flow to that tissue.
Bicarbonate ions are crucial for regulating blood pH. A person's breathing rate influences the level of CO2 in their blood. Breathing that is too slow or shallow causes respiratory acidosis, while breathing that is too rapid leads to hyperventilation, which can cause respiratory alkalosis.
Although the body requires oxygen for metabolism, low oxygen levels do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others — otherwise one risks going unconscious.
The respiratory centers try to maintain an arterial CO2 pressure of 40 mm Hg. With intentional hyperventilation, the CO2 content of arterial blood may be lowered to 10-20 mm Hg (the oxygen content of the blood is little affected), and the respiratory drive is diminished. This is why one can hold one's breath longer after hyperventilating than without hyperventilating. This carries the risk that unconsciousness may result before the need to breath becomes overwhelming, which is why hyperventilation is particularly dangerous before free diving.
Breathing produces approximately 2.3 pounds (1 kg) of carbon dioxide per day per person.
See also
- Bosch reaction
- Carbogen
- Carbon cycle
- Carbon dioxide (data page)
- Carbon dioxide sensor
- Carbon dioxide sink
- Carbon monoxide
- CO2 sequestration
- CO2 degassing in Lake Nyos
- Dry ice
- EcoCute - As refrigerants
- Emission standards
- Global warming
- Greenhouse gas
- Kaya identity
- Ocean acidification
- Sabatier process
References
- Mauna Loa CO2 annual mean data from NOAA. "Trend" data was used. See also: Trends in Carbon Dioxide from NOAA.
- "Annual Mean Growth Rate for Mauna Loa, Hawaii". Trends in Atmospheric Carbon Dioxide. NOAA Earth System Research Laboratory. Retrieved 28 April 2010.
- ^ Toxicity of Carbon Dioxide Gas Exposure, CO2 Poisoning Symptoms, Carbon Dioxide Exposure Limits, and Links to Toxic Gas Testing Procedures By Daniel Friedman - InspectAPedia
- "Carbon Dioxide as a Fire Suppressant: Examining the Risks". U.S. Environmental Protection Agency:.
{{cite news}}: CS1 maint: extra punctuation (link) - Staff (16 August 2006). "Carbon dioxide: IDLH Documentation". National Institute for Occupational Safety and Health. Retrieved 2007-07-05.
- "Phase change data for Carbon dioxide". National Institute of Standards and Technology. Retrieved 2008-01-21.
- Santoro, M.; Gorelli, FA; Bini, R; Ruocco, G; Scandolo, S; Crichton, WA (2006). "Amorphous silica-like carbon dioxide". Nature. 441 (7095): 857–860. doi:10.1038/nature04879. PMID 16778885.
- Priestley, Joseph; Hey, Wm (1772). "Observations on Different Kinds of Air". Philosophical Transactions. 62: 147–264. doi:10.1098/rstl.1772.0021.
- Davy, Humphry (1823). "On the Application of Liquids Formed by the Condensation of Gases as Mechanical Agents". Philosophical Transactions. 113: 199–205. doi:10.1098/rstl.1823.0020.
{{cite journal}}:|format=requires|url=(help) - Duane, H.D. Roller; Thilorier, M. (1952). "Thilorier and the First Solidification of a "Permanent" Gas (1835)". Isis. 43 (2): 109–113. doi:10.1086/349402.
- Strassburger, Julius (1969). Blast Furnace Theory and Practice. New York: American Institute of Mining, Metallurgical, and Petroleum Engineers.
- ^ Pierantozzi, Ronald (2001). "Carbon Dioxide". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. doi:10.1002/0471238961.0301180216090518.a01.pub2.
- Stafford, Ned (2007). "Future crops: The other greenhouse effect". Nature. 448: 7153. doi:10.1038/448526a.
- Clayton, Mark (2006-01-11). "Algae - like a breath mint for smokestacks". Christian Science Monitor. Retrieved 2007-10-11.
- Davidson, Sarah (2005-01-17). "Sweet and environmentally beneficial discovery: Plastics made from orange peel and a greenhouse gas". Cornell News. Retrieved 2007-09-09.
- Austell, J Michael (2005). "CO2 for Enhanced Oil Recovery Needs - Enhanced Fiscal Incentives". Exploration & Production: the Oil & Gas Review. Retrieved 2007-09-28.
- "The Coca-Cola Company Announces Adoption of HFC-Free Insulation in Refrigeration Units to Combat Global Warming". The Coca-Cola Company. 2006-06-05. Retrieved 2007-10-11.
- "Modine reinforces its CO2 research efforts". R744.com. 2007-06-28.
- "Enhanced coal bed methane recovery". ETH Zurich. 2006-08-31.
- NASA Earth Fact Sheet
- Dr. Pieter Tans (3 May 2008) "Annual CO2 mole fraction increase (ppm)" for 1959-2007 National Oceanic and Atmospheric Administration Earth System Research Laboratory, Global Monitoring Division (additional details.)
- "Climate and CO2 in the Atmosphere". Retrieved 2007-10-10.
- Berner, Robert A.; Kothavala, Zavareth (2001). "GEOCARB III: A Revised Model of Atmospheric CO2 over Phanerozoic Time" (PDF). American Journal of Science. 301: 182–204. doi:10.2475/ajs.301.2.182. Retrieved 2008-02-15.
- "After two large annual gains, rate of atmospheric CO2 increase returns to average". NOAA News Online, Story 2412. 2005-03-31.
- Sigurdsson, Haraldur (2000). Encyclopedia of volcanoes. San Diego: Academic Press. ISBN 012643140X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - van Gardingen, P.R. (1997). "Long-term effects of enhanced CO2 concentrations on leaf gas exchange: research opportunities using CO2 springs". In Raschi, A.; Miglietta, F.; Tognetti, R.; van Gardingen, P.R. (Eds.) (ed.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 0521582032.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: multiple names: editors list (link) - Martini, M. (1997). "CO2 emissions in volcanic areas: case histories and hazaards". In Raschi, A.; Miglietta, F.; Tognetti, R.; van Gardingen, P.R. (Eds.) (ed.). Plant responses to elevated CO2: Evidence from natural springs. Cambridge: Cambridge University Press. pp. 69–86. ISBN 0521582032.
{{cite book}}: CS1 maint: multiple names: editors list (link) - "Volcanic Gases and Their Effects". Retrieved 2007-09-07.
- Doney, Scott C. (2006-11-29). "How Long Can the Ocean Slow Global Warming?". Oceanus. Retrieved 2007-11-21.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Duana, Zhenhao (2003). "An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar". Chemical Geology. 193: 260–271.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Garrison, Tom (2004). Oceanography: An Invitation to Marine Science. Thomson Brooks. p. 125. ISBN 0534408877.
- Ries, Justin B. (2009-12-01). "Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification". Geology.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Climate Change 2007: Synthesis Report, IPCC
- PMEL Ocean Acidification Home Page
- Blom, T.J. (2002-12). "Carbon Dioxide In Greenhouses". Retrieved 2007-06-12.
{{cite web}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Global Warming? What a load of poppycock! by Professor David Bellamy Daily Mail, July 9, 2004
- F. Woodward and C. Kelly (1995). "The influence of CO2 concentration on stomatal density". New Phytologist. 131: 311–327. doi:10.1111/j.1469-8137.1995.tb03067.x.
- Bert G. Drake; Gonzalez-Meler, Miquel A.; Long, Steve P. (1997). "More efficient plants: A Consequence of Rising Atmospheric CO2?". Annual Review of Plant Physiology and Plant Molecular Biology. 48: 609. doi:10.1146/annurev.arplant.48.1.609.
- Loladze, I (2002). "Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry?". Trends in Ecology & Evolution. 17: 457. doi:10.1016/S0169-5347(02)02587-9.
- Carlos E. Coviella and John T. Trumble (1999). "Effects of Elevated Atmospheric Carbon Dioxide on Insect-Plant Interactions". Conservation Biology. 13 (4): 700.
- "Global Environment Division Greenhouse Gas Assessment Handbook - A Practical Guidance Document for the Assessment of Project-level Greenhouse Gas Emissions". World Bank. Retrieved 2007-11-10.
- Luyssaert, Sebastiaan; Schulze, E. -Detlef; Börner, Annett; Knohl, Alexander; Hessenmöller, Dominik; Law, Beverly E.; Ciais, Philippe; Grace, John (2008). "Old-growth forests as global carbon sinks". Nature. 455: 213. doi:10.1038/nature07276.
- Falkowski, P.; Scholes, RJ; Boyle, E; Canadell, J; Canfield, D; Elser, J; Gruber, N; Hibbard, K; Högberg, P (2000). "The global carbon cycle: a test of our knowledge of earth as a system". Science. 290 (5490): 291–296. doi:10.1126/science.290.5490.291. PMID 11030643.
- ^ Davidson, Clive. 7 February 2003. "Marine Notice: Carbon Dioxide: Health Hazard". Australian Maritime Safety Authority.
- "Graphical map of CO2".
- Gowda Shilpa (2 November 2007). "New Insight into Panic Attacks: Carbon Dioxide is the Culprit".
- "Inhaled carbon dioxide increases brain acidity and evokes fear behavior". 26 November 2009.
- Occupational Safety and Health Administration. Chemical Sampling Information: Carbon Dioxide. Retrieved 5 June 2008 from: http://www.osha.gov/dts/chemicalsampling/data/CH_225400.html
- ^ Glatte Jr H. A., Motsay G. J., Welch B. E. (1967). "Carbon Dioxide Tolerance Studies". Brooks AFB, TX School of Aerospace Medicine Technical Report. SAM-TR-67-77. Retrieved 2008-05-02.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lambertsen, C. J. (1971). "Carbon Dioxide Tolerance and Toxicity". Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center. IFEM Report No. 2-71. Philadelphia, PA. Retrieved 2008-05-02.
- How are people able to breathe inside a submarine?
- ^ "Carbon dioxide". solarnavigator.net. Retrieved 2007-10-12.
- "How much carbon dioxide do humans contribute through breathing?". Retrieved 2009-04-30.
Shendell, Prill, Fisk, Apte1, Blake & Faulkner, Associations between classroom CO2 concentrations and student attendance in Washington and Idaho, Indoor Air 2004. SEPPANEN, FISK AND MENDELL, Association of Ventilation Rates and CO2 Concentrations with Health and Other Responses in Commercial and Institutional Buildings, Indoor Air 1999.
Further reading
- Tyler Volk (2008), CO2 Rising: The World's Greatest Environmental Challenge, The MIT Press, 223 pages, ISBN 978-0262220835. A short, balanced primer on CO2's role as a greenhouse gas. Review at Environmental Health Perspectives
External links
- International Chemical Safety Card 0021
- CID 280 from PubChem
- CO2 Carbon Dioxide Properties, Uses, Applications
- Dry Ice information
- Trends in Atmospheric Carbon Dioxide (NOAA)
- NASA's Orbiting Carbon Observatory
- The on-line catalogue of CO2 natural emissions in Italy
| Oxocarbons | |
|---|---|
| Common oxides | |
| Exotic oxides | |
| Polymers | |
| Compounds derived from oxides | |