| Revision as of 14:22, 27 December 2010 editWickey-nl (talk | contribs)Extended confirmed users7,037 editsNo edit summary← Previous edit | Revision as of 16:55, 3 January 2011 edit undoWickey-nl (talk | contribs)Extended confirmed users7,037 edits imageNext edit → | ||
| Line 1: | Line 1: | ||
| In ], a '''cyclic compound''' is a, mostly organic, ] in which a series of atoms is connected to form a loop or ring.<ref>{{JerryMarch}}</ref> | In ], a '''cyclic compound''' is a, mostly organic, ] in which a series of atoms is connected to form a loop or ring.<ref>{{JerryMarch}}</ref> | ||
| Cyclic compounds may or may |
Cyclic compounds may or may not be ]. ] is a well known example. The term "polycyclic" is used when more than one ring is formed in a single molecule for instance in ], and the term ] is used for a ring containing more than a dozen atoms. | ||
| <gallery> | <gallery> | ||
| Image: |
Image:cycloheptane sticks.png|], a non-aromatic cyclic compound. | ||
| Image: |
Image:Benzene bonds.svg|], a cyclic compound. | ||
| Image: |
Image:Naphthalene.png|], a polycyclic compound. | ||
| Image:Porphyrin.svg|], a macrocyclic compound. | |||
| File:Pentazole.png|], an inorganic cyclic compound. | File:Pentazole.png|], an inorganic cyclic compound. | ||
| </gallery> | </gallery> | ||
Revision as of 16:55, 3 January 2011
In chemistry, a cyclic compound is a, mostly organic, compound in which a series of atoms is connected to form a loop or ring. Cyclic compounds may or may not be aromatic. Benzene is a well known example. The term "polycyclic" is used when more than one ring is formed in a single molecule for instance in naphthalene, and the term macrocycle is used for a ring containing more than a dozen atoms.
-
 Cycloheptane, a non-aromatic cyclic compound.
Cycloheptane, a non-aromatic cyclic compound.
-
 Benzene, a cyclic compound.
Benzene, a cyclic compound.
-
 Naphthalene, a polycyclic compound.
Naphthalene, a polycyclic compound.
-
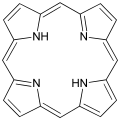 Porphyrin, a macrocyclic compound.
Porphyrin, a macrocyclic compound.
-
 Pentazole, an inorganic cyclic compound.
Pentazole, an inorganic cyclic compound.
Categorization
Cyclic compounds can be categorized:
Ring-closing & opening reactions

Related concepts in organic chemistry are so-called ring-closing reactions in which a cyclic compound is formed and ring-opening reactions in which rings are opened.
Examples of ring-closing reactions:
- Ring-closing metathesis
- Nazarov cyclization reaction
- Ruzicka large ring synthesis
- Dieckmann condensation
- Wenker synthesis
- Radical cyclization
Example of ring-opening reactions:
- A general type of polymerization reaction: Ring-opening polymerization
- Ring opening metathesis polymerisation
See also
- open-chain compound
- Ring expansion and ring contraction
- Macrocycle
- Cyclization reaction
- Effective molarity
External links
- Polycyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Macrocyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
References
- March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.