| Revision as of 20:49, 2 September 2011 editBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot← Previous edit | Revision as of 21:03, 2 September 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'DrugBank_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_Pharmacology|Next edit → | ||
| Line 1: | Line 1: | ||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 413289716 | ||
| | IUPAC_name = (6aR,10aR)-3-(1-hexylcyclopropyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzochromen-1-ol | | IUPAC_name = (6aR,10aR)-3-(1-hexylcyclopropyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzochromen-1-ol | ||
| | image = AMG-41.svg | | image = AMG-41.svg | ||
Revision as of 21:03, 2 September 2011
Pharmaceutical compound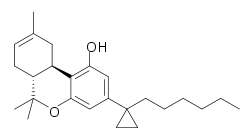 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H36O2 |
| Molar mass | 368.551 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
AMG-41 is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8THC substituted with a cyclopropane group on the 3-position side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.4nM at CB1 vs 0.9nM at CB2.
References
- Papahatjis DP, Nikas SP, Andreou T, Makriyannis A. Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols. Bioorganic and Medicinal Chemistry Letters. 2002 Dec 16;12(24):3583-6. PMID 12443781
- Papahatjis DP, Nikas SP, Kourouli T, Chari R, Xu W, Pertwee RG, Makriyannis A. Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'. Journal of Medicinal Chemistry. 2003 Jul 17;46(15):3221-9. PMID 12852753
- Papahatjis DP, Nahmias VR, Nikas SP, Andreou T, Alapafuja SO, Tsotinis A, Guo J, Fan P, Makriyannis A. C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols. Journal of Medicinal Chemistry. 2007 Aug 23;50(17):4048-60. PMID 17672444
This cannabinoid related article is a stub. You can help Misplaced Pages by expanding it. |