| Revision as of 03:32, 28 March 2006 edit70.67.216.192 (talk) →Life cycle← Previous edit | Revision as of 20:30, 28 March 2006 edit undo204.244.150.7 (talk) →Laboratory diagnosisNext edit → | ||
| Line 73: | Line 73: | ||
| ] | ] | ||
| The diagnosis rests upon demonstrating trypanosomes by microscopic examination of chancre fluid, lymph node aspirates, blood, bone marrow, or, in the late stages of infection, cerebrospinal fluid. A wet preparation should be examined for the motile trypanosomes, and in addition a smear should be fixed, stained with Giemsa (or Field), and examined. Concentration techniques can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the ]; mini anion-exchange/centrifugation; and the Quantitative Buffy Coat (QBC) technique. For other samples such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment. Isolation of the parasite by inoculation of rats or mice is a sensitive method, but its use is limited to ''T. b. rhodesiense''. Antibody detection has sensitivity and specificity that are too variable for clinical decisions. In addition, in infections with ''T. b. rhodesiense'', seroconversion occurs after the onset of clinical symptoms and thus is of limited use. | The diagnosis rests upon demonstrating trypanosomes by microscopic examination of chancre fluid, lymph node aspirates, blood, bone marrow, or, in the late stages of infection, cerebrospinal fluid. A wet preparation should be examined for the motile trypanosomes, and in addition a smear should be fixed, stained with Giemsa (or Field), and examined. Concentration techniques can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the ]; mini anion-exchange/centrifugation; and the Quantitative Buffy Coat (QBC) technique. For other samples such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment. Isolation of the parasite by inoculation of rats or mice is a sensitive method, but its use is limited to ''T. b. rhodesiense''. Antibody detection has sensitivity and specificity that are too variable for clinical decisions. In addition, in infections with ''T. b. rhodesiense'', seroconversion occurs after the onset of clinical symptoms and thus is of limited use.ddddddd | ||
| ==Treatment== | ==Treatment== | ||
Revision as of 20:30, 28 March 2006
Medical condition| African trypanosomiasis | |
|---|---|
| Specialty | Infectious diseases |
Sleeping sickness or African trypanosomiasis is a parasitic disease in people and in animals. Caused by protozoa of genus Trypanosoma and transmitted by the tsetse fly, the disease is endemic in certain regions of Sub-Saharan Africa, covering about 36 countries and 60 million people. It is estimated that 300,000 - 500,000 people are infected, and about 40,000 die every year. Three major epidemics have occurred in the past hundred years, in 1896 - 1906, 1920, and 1970.
History
The condition has been present in Africa from at least the 14th century. The causative agent and the vector were not identified until 1902 - 1903 by Sir David Bruce, and the differentiation between protozoa was not made until 1910. Atoxyl, an arsenic based drug developed by Paul Ehrlich and Kiyoshi Shiga in 1906(?), was the first effective drug, but blindness was a serious side effect. In 1920 Suramin was introduced to treat the first stage of the disease, later on it was generally combined with tryparsamide in the treatment of the second stage. In 1922 Tryparsamide, another pentavalent organo-arsenic drug entered the market. It is effective in the second stage of the disease of the gambiense form. It was used during the grand epidemic in West and Central Africa in millions of people and was the mainstay of therapy until 1969.
Pentamidine, a highly effective drug for the first stage of the disease, has been used since 1939. During the fifties, it was widely used as a prophylactic agent in Western Africa, leading to a sharp decline in infection rates. At the time, it was thought that eradication of the disease was at hand.
The organo-arsenical melarsoprol (Arsobal) was developed in the 1940s, and is effective for patients with second stage sleeping sickness. However, 3 - 10% of those injected have reactive encephalopathy (convulsions, progressive coma, or psychotic reactions), and 10 - 70% die; it can cause brain damage in those that survive the encephalopathy. However, due to its effectiveness, melarsoprol is still used today.
There have been three severe epidemics in Africa over the last century: one between 1896 and 1906, mostly in Uganda and the Congo Basin, one in 1920 in several African countries, and one that began in 1970 and is still in progress. The 1920 epidemic was arrested due to mobile teams systematically screening millions of people at risk. The disease had practically disappeared between 1960 and 1965. After that success, screening and effective surveillance were relaxed, and the disease has reappeared in endemic form in several foci over the last thirty years.
The British statesman, Lord Alfred Milner died of sleeping sickness in 1923, shortly after returning from a visit to South Africa.
Geographic distribution and epidemiology
The disease is found in two forms, depending on the parasite, either Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense. T. b. gambiense is found in central and western Africa; it causes a chronic condition that can extend in a passive phase for months or years before symptoms emerge. T. b. rhodesiense, is the acute form of the disease but has a much more limited range. It is found in southern and eastern Africa; its infection emerges in a few weeks and is more virulent and faster developing. According to recent estimates, the disability adjusted life years (DALYs) lost due to sleeping sickness are 2.0 million. Recent estimates indicate that over 60 million people living in some 250 foci are at risk of contracting the disease, and there are about 300,000 new cases each year. The disease has been recorded as occurring in 36 countries, all in sub-Saharan Africa.
Humans are the main reservoir for Trypanosoma brucei gambiense, but this species can also be found in pigs and other animals. Wild game animals and cattle are the main reservoir of T. b. rhodesiense.
Horse-flies (Tabanidae) and Stomoxydinae possibly could play a role by mechanical transmission (in special situations) not only of Nagana.
Life cycle
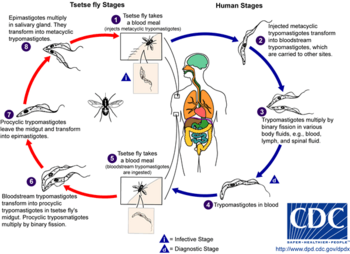
During a blood meal on the mammalian host, an infected tsetse fly (genus Glossina) injects metacyclic trypomastigotes into skin tissue. The parasites enter the lymphatic system and pass into the bloodstream (1). Inside the host, they transform into bloodstream trypomastigotes (2), are carried to other sites throughout the body, reach other blood fluids (e.g., lymph, spinal fluid), and continue the replication by binary fission (3). The entire life cycle of African Trypanosomes is represented by extracellular stages. A tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host (4,5). In the fly's midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission (6), leave the midgut, and transform into epimastigotes (7). The epimastigotes reach the fly's salivary glands and continue multiplication by binary fission (8). The cycle in the fly takes approximately 3 weeks to digest.bye
Clinical features
Symptoms begin with fever, headaches, and joint pains. If untreated, the disease slowly overcomes the defenses of the infected person, and symptoms spread to anaemia, endocrine problems, and cardiovascular and kidney disorders. The disease then enters a neurological phase when the parasite passes through the blood-brain barrier. The symptoms of the second phase is what gives the disease its name: besides confusion and reduced coordination, the sleep cycle is disturbed with bouts of fatigue punctuated with manic periods progressing to daytime slumber and nighttime insomnia. Without treatment, the disease is fatal, with progressive mental deterioration leading to coma and death. Damage caused in the neurological phase can be irreversible.
In addition to the bite of the tsetse fly, the disease is contractable in the following ways:
- Mother to child infection: the trypanosome can cross the placenta and infect the fetus, causing abortion and perinatal death.
- Laboratories: accidental infections, for example, through the handling of blood of an infected person, although this is uncommon.
Laboratory diagnosis

The diagnosis rests upon demonstrating trypanosomes by microscopic examination of chancre fluid, lymph node aspirates, blood, bone marrow, or, in the late stages of infection, cerebrospinal fluid. A wet preparation should be examined for the motile trypanosomes, and in addition a smear should be fixed, stained with Giemsa (or Field), and examined. Concentration techniques can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the buffy coat; mini anion-exchange/centrifugation; and the Quantitative Buffy Coat (QBC) technique. For other samples such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment. Isolation of the parasite by inoculation of rats or mice is a sensitive method, but its use is limited to T. b. rhodesiense. Antibody detection has sensitivity and specificity that are too variable for clinical decisions. In addition, in infections with T. b. rhodesiense, seroconversion occurs after the onset of clinical symptoms and thus is of limited use.ddddddd
Treatment
Eflornithine (difluoromethylornithine or DFMO), the most modern treatment, was developed in the 1970s by Albert Sjoerdsmanot and underwent clinical trials in the 1980s. The drug was approved by the FDA in 1990, but Aventis, the company responsible for its manufacture, halted production in 1999. In 2001, however, Aventis, in association with Medecins Sans Frontieres and the WHO, signed a long-term agreement to manufacture and donate the drug.
The genome of the parasite has been decoded and several proteins have been identified as potential targets for drug treatment. The decoded DNA also revealed the reason that generating a vaccine for this disease has been so difficult. T. burcei has over 800 genes that manufacture proteins that the disease mixes and matches to evade immune system detection. (Berriman, et al., 2005)
The primary condition is typically treated with either suramin (T. b. rhodesiense/gambiense) or pentamidine (T. b. gambiense). Advanced cases can be treated with melarsoprol or eflornithine. All these drugs, especially melarsoprol, have many undesirable side-effects, and the treatment regimen is often difficult to enforce.
An international research team working in the Democratic Republic of the Congo, New Sudan and Angola involving Immtech International and University of North Carolina at Chapel Hill have completed a Phase IIb clinical trial and commenced a Phase III trial in 2005 testing the efficacy of the first oral treatment for Sleeping Sickness, known at this point as "DB289".
Prevention and control
Prevention and control focus on, where it is possible, the eradication of the parasitic host, the tsetse fly. Two alternative strategies have been used in the attempts to reduce the African trypanosomiases. One tactic is primarily medical or veterinary and targets the disease directly using monitoring, prophylaxis, treatment, and surveillance to reduce the number of organisms which carry the disease. The second strategy is generally entomological and intends to disrupt the cycle of transmission by reducing the number of flies. For in depth information on prevention of the disease via tse tse fly control see Tsetse fly control
Instances of sleeping sickness are being reduced by the use of the Sterile Atomic Fly.
Regular active surveillance, involving case detection and treatment, in addition to tsetse fly control, is the backbone of the strategy for control of sleeping sickness. Systematic screening of communities in identified foci is the best approach as case-by-case screening is not practically possible in highly endemic regions. Systematic screening may be in the form of mobile clinics or fixed screening centres where teams travel daily to the foci. The nature of gambiense disease is such that patients don't seek treatment early enough because the symptoms at that stage are not evident or serious enough to warrant seeking medical attention, considering the remoteness of some affected areas. Also, diagnosis of the disease is difficult and most health workers may not be able to detect it. Systematic screening allows early-stage disease to be detected and treated before the disease progresses, and removes the potential human reservoir.
See also
- David Bruce (microbiologist)
- Sleep disorder
- The other important human tropical disease caused by Trypanosomes is Chagas-disease, occurring in America.
Recent news and events
- This illness was featured in an episode of House titled "Fidelity", aired 2004-12-28.
- 2006: Nature.com / Scott M. Landfear: Flagella are whip-like structures that power the movement of certain cells. Analysis of a single-cell parasite, the African trypanosome, reveals that flagella are also essential for viability in this organism.
References
- Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-Sayed NM (2005). "The genome of the African trypanosome Trypanosoma brucei". Science. 309 (5733): 416–22. PMID 16020726.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Numbered references
- http://www.who.int/mediacentre/factsheets/fs259/en/index.html.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help); Unknown parameter|Author=ignored (|author=suggested) (help); Unknown parameter|PublishYear=ignored (help); Unknown parameter|Title=ignored (|title=suggested) (help) - http://www.who.int/tdr/diseases/tryp/direction.htm#Refs.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help); Unknown parameter|Author=ignored (|author=suggested) (help); Unknown parameter|PublishYear=ignored (help); Unknown parameter|Title=ignored (|title=suggested) (help) - http://www.who.int/tdr/diseases/tryp/direction.htm#Refs.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help); Unknown parameter|Author=ignored (|author=suggested) (help); Unknown parameter|PublishYear=ignored (help); Unknown parameter|Title=ignored (|title=suggested) (help) - Cherenet T, Sani RA, Panandam JM, Nadzr S, Speybroeck N, van den Bossche P (2004). "Seasonal prevalence of bovine trypanosomosis in a tsetse-infested zone and a tsetse-free zone of the Amhara Region, north-west Ethiopia". The Onderstepoort journal of veterinary research. 71 (4): 307–312.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Williamson, David (August 25, 2005). "Compound might defeat African sleeping sickness, clinical trial beginning this month". University of North Carolina.
- Staff (September 15, 2005). "Clinical Trials Update". Genetic Engineering News. p. 5.
- "Strategic Direction for African Trypanosomiasis Research". Special Programme for Research and Training in Tropical Diseases. Retrieved 2006-03-01.
External links
- Doctors Without Borders/Medecins Sans Frontieres Sleeping sickness information page
- Medecins Sans Frontieres' Eflornithine press release, 2001
- Links to pictures of Sleeping Sickness (Hardin MD/Univ of Iowa)