| Revision as of 19:30, 7 December 2011 editEdgar181 (talk | contribs)Extended confirmed users196,325 edits no longer a stub← Previous edit | Revision as of 15:22, 19 December 2011 edit undoEmausBot (talk | contribs)Bots, Template editors2,862,818 editsm r2.6.4) (Robot: Modifying el:1-βουτένιοNext edit → | ||
| Line 67: | Line 67: | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
Revision as of 15:22, 19 December 2011
 | |||
| |||
| Names | |||
|---|---|---|---|
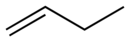
| IUPAC name but-1-ene | |||
| Other names ethylethylene, 1-butylene, α-butylene, but-1-ene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.137 | ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H8 | ||
| Molar mass | 56.108 g·mol | ||
| Appearance | Colorless Gas | ||
| Density | 2.37 g/dm | ||
| Melting point | −185 °C (−301.0 °F; 88.1 K) | ||
| Boiling point | -6.3 °C, 266.9 K, 20.7 °F | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
1-Butene is an organic chemical compound, linear alpha-olefin (alkene), and one of the isomers of butene. The formula is C
4H
8.
Stability
1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air. It is, however, incompatible with metal salts, fluorine and other halogens, nitrogen oxides, boron trifluoride, hydrohalic acids, and strong oxidizing agents.
Manufacturing
1-butene is produced either by separation from crude C4 refinery streams or from the reaction of ethylene. It is distilled to give a very high-purity product. 1-butene is used to manufacture lots of other chemical products, such as linear low-density polyethylene (LLDPE), polypropylene resins, polybutene, butylene oxide, and methyl ethyl ketone (MEK).