| Revision as of 18:03, 7 May 2011 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,331 edits cat repair← Previous edit | Revision as of 04:41, 25 December 2011 edit undoCitation bot 1 (talk | contribs)Bots130,044 editsm Add: issue, pmid. Tweak: issue, pages. Formatted dashes. You can use this bot yourself. Report bugs here.Next edit → | ||
| Line 36: | Line 36: | ||
| }} | }} | ||
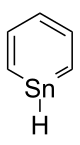
| '''Stannabenzene''' (C<sub>5</sub>H<sub>6</sub>Sn) is the parent representative of a group of ]s that are related to ] with a ] atom replaced by a ] atom. Stannabenzene itself has been studied by ],<ref>{{cite journal | doi = 10.1016/j.theochem.2009.10.038 | author = Ebrahimi, Arash Afshar; Ghiasi, Reza; Foroutan-Nejad, Cina | title = Topological characteristics of the ring critical points and the aromaticity of groups IIIA to VIA hetero-benzenes | journal = Journal of Molecular Structure: THEOCHEM | year = 2010 | volume = 941 | issue = |
'''Stannabenzene''' (C<sub>5</sub>H<sub>6</sub>Sn) is the parent representative of a group of ]s that are related to ] with a ] atom replaced by a ] atom. Stannabenzene itself has been studied by ],<ref>{{cite journal | doi = 10.1016/j.theochem.2009.10.038 | author = Ebrahimi, Arash Afshar; Ghiasi, Reza; Foroutan-Nejad, Cina | title = Topological characteristics of the ring critical points and the aromaticity of groups IIIA to VIA hetero-benzenes | journal = Journal of Molecular Structure: THEOCHEM | year = 2010 | volume = 941 | issue = 1–3 | pages = 47–52}}</ref> but has not been isolated. | ||
| ==Stable derivatives of stannabenzene== | ==Stable derivatives of stannabenzene== | ||
| Stable derivatives of stannabenzene are have been isolated. The 2-stannanaphthalene depicted below is stable in an inert atmosphere at temperatures below 140 °C.<ref>{{cite journal | last1 = Mizuhata | first1 = Yoshiyuki | last2 = Sasamori | first2 = Takahiro | last3 = Takeda | first3 = Nobuhiro | last4 = Tokitoh | first4 = Norihiro | title = A Stable Neutral Stannaaromatic Compound: Synthesis, Structure and Complexation of a Kinetically Stabilized 2-Stannanaphthalene | journal = Journal of the American Chemical Society | volume = 128 | pages = |
Stable derivatives of stannabenzene are have been isolated. The 2-stannanaphthalene depicted below is stable in an inert atmosphere at temperatures below 140 °C.<ref>{{cite journal | last1 = Mizuhata | first1 = Yoshiyuki | last2 = Sasamori | first2 = Takahiro | last3 = Takeda | first3 = Nobuhiro | last4 = Tokitoh | first4 = Norihiro | title = A Stable Neutral Stannaaromatic Compound: Synthesis, Structure and Complexation of a Kinetically Stabilized 2-Stannanaphthalene | journal = Journal of the American Chemical Society | volume = 128 | pages = 1050–1 | year = 2006 | doi = 10.1021/ja057531d | issue = 4 | pmid = 16433501}}</ref> The tin to carbon bond in this compound is shielded from potential reactants by two very bulky groups, one ] group and the even larger 2,4,6-trisphenyl or Tbt group. The two Sn-C bonds have ]s of 202.9 and 208.1 ] which are shorter than those for Sn-C single bonds (214 pm) and comparable to that of known Sn=C ]s (201.6 pm). The C-C bonds show little variation with bond lengths between 135.6 and 144.3 pm signaling that this compound is ]. | ||
| :]{{clear-left}} | :]{{clear-left}} | ||
Revision as of 04:41, 25 December 2011
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Stannine | |||
| Other names Stannin | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H6Sn | ||
| Molar mass | 184.813 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated.
Stable derivatives of stannabenzene
Stable derivatives of stannabenzene are have been isolated. The 2-stannanaphthalene depicted below is stable in an inert atmosphere at temperatures below 140 °C. The tin to carbon bond in this compound is shielded from potential reactants by two very bulky groups, one tert-butyl group and the even larger 2,4,6-trisphenyl or Tbt group. The two Sn-C bonds have bond lengths of 202.9 and 208.1 pm which are shorter than those for Sn-C single bonds (214 pm) and comparable to that of known Sn=C double bonds (201.6 pm). The C-C bonds show little variation with bond lengths between 135.6 and 144.3 pm signaling that this compound is aromatic.
Tbt-substituted 9-stannaphenanthrene was reported in 2005 . At room temperature it forms the cycloadduct.
Tbt-substituted stannabenzene was reported in 2010.. At room-temperature it quantitatively forms the DA dimer.

Tbt-substituted stannabenzene synthesis. Reagents lithium aluminum hydride (step 2), NBS (step 3), LDA (step 4)
See also
- Other 6-membered aromatic rings with one carbon replaced by another group: borabenzene, benzene, silabenzene, germabenzene, pyridine, phosphorine, pyrylium salt
References
- Ebrahimi, Arash Afshar; Ghiasi, Reza; Foroutan-Nejad, Cina (2010). "Topological characteristics of the ring critical points and the aromaticity of groups IIIA to VIA hetero-benzenes". Journal of Molecular Structure: THEOCHEM. 941 (1–3): 47–52. doi:10.1016/j.theochem.2009.10.038.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mizuhata, Yoshiyuki; Sasamori, Takahiro; Takeda, Nobuhiro; Tokitoh, Norihiro (2006). "A Stable Neutral Stannaaromatic Compound: Synthesis, Structure and Complexation of a Kinetically Stabilized 2-Stannanaphthalene". Journal of the American Chemical Society. 128 (4): 1050–1. doi:10.1021/ja057531d. PMID 16433501.
{{cite journal}}: no-break space character in|title=at position 42 (help) - Generation of 9-Stannaphenanthrene and Its Reactivities Yoshiyuki Mizuhata, Nobuhiro Takeda, Takahiro Sasamori and Norihiro Tokitoh Chemistry Letters Volume 34 Number 8 Year 2005 Page 1088 doi:10.1246/cl.2005.1088
- Generation of Stannabenzenes and Their Properties Yoshiyuki Mizuhata, Naoya Noda, and Norihiro Tokitoh Organometallics, 2010, 29 (21), pp 4781–4784 doi:10.1021/om100382n