| Revision as of 02:29, 25 December 2011 editCitation bot 1 (talk | contribs)Bots130,044 editsm Add: issue. You can use this bot yourself. Report bugs here.← Previous edit | Revision as of 03:50, 27 December 2011 edit undoShalalawudi (talk | contribs)2 editsNo edit summaryNext edit → | ||
| Line 52: | Line 52: | ||
| : Ni(COD)<sub>2</sub> + 4 P(OC<sub>6</sub>H<sub>5</sub>)<sub>3</sub> → Ni<sub>4</sub> + 2 COD | : Ni(COD)<sub>2</sub> + 4 P(OC<sub>6</sub>H<sub>5</sub>)<sub>3</sub> → Ni<sub>4</sub> + 2 COD | ||
| It also forms a variety of Fe(O) and Fe(II) complexes such as the di] H<sub>2</sub>Fe<sub>4</sub>.<ref>{{cite journal|doi=10.1021/ja00768a022|title=Stereochemically nonrigid six-coordinate molecules. II. Preparations and reactions of tetrakis(organophosphorus)metal dihydride complexes|year=1972|last1=Gerlach|first1=D. H.|last2=Peet|first2=W. G.|last3=Muetterties|first3=E. L.|journal=Journal of the American Chemical Society|volume=94|pages=4545|issue=13}}</ref> | It also forms a variety of Fe(O) and Fe(II) complexes such as the di] H<sub>2</sub>Fe<sub>4</sub>.<ref>{{cite journal|doi=10.1021/ja00768a022|title=Stereochemically nonrigid six-coordinate molecules. II. Preparations and reactions of tetrakis(organophosphorus)metal dihydride complexes|year=1972|last1=Gerlach|first1=D. H.|last2=Peet|first2=W. G.|last3=Muetterties|first3=E. L.|journal=Journal of the American Chemical Society|volume=94|pages=4545|issue=13}}</ref><ref></ref> | ||
| ==References== | ==References== | ||
Revision as of 03:50, 27 December 2011
| |

| |
| Names | |
|---|---|
| IUPAC name Triphenyl phosphite | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.002.645 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H15O3P |
| Molar mass | 310.28 g/mol |
| Appearance | colourless liquid |
| Density | 1.184 g/mL |
| Melting point | 22–24 °C |
| Boiling point | 360 °C |
| Solubility in water | organic solvents |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | flammable |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
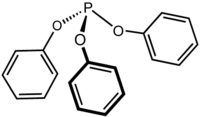
Triphenyl phosphite is the chemical compound with the formula P(OC6H5)3. This colourless viscous liquid is the ester of phosphorous acid and phenol. It is used as a ligand in organometallic chemistry. Nickel complexes of this ligand are homogeneous catalysts for the hydrocyanation of alkenes.
Triphenylphosphite is prepared from phosphorus trichloride and phenol in the presence of a base:
- PCl3 + 3 HOC6H5 → P(OC6H5)3 + 3 HCl
Trimethylphosphine is prepared from triphenylphosphite:
- 3 CH3MgBr + P(OC6H5)3 → P(CH3)3 + 3 MgBrOC6H5
Triphenylphosphite is a notable example of polyamorphism in organic compounds, namely it exists in two different amorphous forms at temperatures about 200 K.
Representative coordination complexes
Triphenylphosphite forms zero-valent complexes of the type M4 for M = Ni, Pd, Pt. The colourless nickel complex (melting point 147 °C) can be prepared from the nickel(0) complex of 1,5-cyclooctadiene:
- Ni(COD)2 + 4 P(OC6H5)3 → Ni4 + 2 COD
It also forms a variety of Fe(O) and Fe(II) complexes such as the dihydride H2Fe4.
References
- Leutkens, Jr., M. L.; Sattelberger, A. P.; Murray, H. H.; Basil, J. D.; Fackler, Jr., J. P. "Trimethylphosphine" Inorganic Syntheses, 1990, volume 28, pages 305-310. ISBN 0-471-52619-3
- Ha, Alice; Cohen, Itai; Zhao, Xiaolin; Lee, Michelle; Kivelson, Daniel (1996). "Supercooled Liquids and Polyamorphism†". The Journal of Physical Chemistry. 100: 1. doi:10.1021/jp9530820.
- Ittel, S. D. "Olefin, Acetylene, Phosphine, Isocyanide, and Diazene Complexes of Nickel(0)" Inorganic Syntheses, 1977, volume XVII, p. 117–124. ISBN 0-07-044327-0,
- Gerlach, D. H.; Peet, W. G.; Muetterties, E. L. (1972). "Stereochemically nonrigid six-coordinate molecules. II. Preparations and reactions of tetrakis(organophosphorus)metal dihydride complexes". Journal of the American Chemical Society. 94 (13): 4545. doi:10.1021/ja00768a022.
- Triphenyl phosphite-Guidechem.com