| Revision as of 12:45, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473097278 of page Cobalt(II)_acetate for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 12:45, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473097283 of page Chromium_hexacarbonyl for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| ⚫ | | verifiedrevid = 443522898 | ||
| | Watchedfields = changed | |||
| | Name = Chromium hexacarbonyl | |||
| ⚫ | | verifiedrevid = |
||
| | ImageFile = |
| ImageFile = Cr(CO)6.png | ||
| | ImageSize = | | ImageSize = 150px | ||
| | ImageName = |
| ImageName = | ||
| | IUPACName = |
| IUPACName = Hexacarbonylchromium | ||
| | OtherNames = | | OtherNames = Chromium carbonyl | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = |
| ChemSpiderID = 23855 | ||
| | InChI = 1/6CO.Cr/c6*1-2; | |||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | UNII = 3XC4P44U7E | |||
| | ChEBI = 33031 | |||
| | InChI = 1/2C2H4O2.Co/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2 | |||
| | SMILES = .#.#.#.#.#.# | |||
| | InChIKey = QAHREYKOYSIQPH-NUQVWONBAX | |||
| | InChIKey = KOTQLLUQLXWWDK-UHFFFAOYAN | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/6CO.Cr/c6*1-2; | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = KOTQLLUQLXWWDK-UHFFFAOYSA-N | ||
| | CASNo = |
| CASNo = 13007-92-6 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | PubChem = 518677 | |||
| | CASOther = (anhydrous)<br/>6147-53-1 (tetrahydrate) | |||
| | |
| RTECS = GB5075000 | ||
| ⚫ | }} | ||
| | SMILES = .C(=O)C.C(=O)C | |||
| ⚫ | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = C<sub>6</sub>CrO<sub>6</sub> | |||
| | Appearance = Pink crystals (anhydrous) <br> intense red crystals (tetrahydrate) | |||
| | MolarMass = 220.057 g/mol | |||
| | Odor = vinegar (tetrahydrate) | |||
| | Appearance = colorless crystals | |||
| | Density = 1. |
| Density = 1.77 g/cm<sup>3</sup>, solid | ||
| | MolarMass = 177.02124 g/mol (anhydrous) <br> 249.08 g/mol (tetrahydrate) | |||
| | Solubility = insoluble | |||
| | MeltingPt = 140 °C (tetrahydrate) | |||
| | SolubleOther = soluble in ], ], ] (THP), ] | |||
| ⚫ | | BoilingPt = | ||
| | |
| MeltingPt = 150 °C | ||
| ⚫ | | BoilingPt = 210 °C (decomp) | ||
| | SolubleOther = soluble in ], dilute acids, ] (tetrahydrate) | |||
| ⚫ | }} | ||
| | RefractIndex = 1.542 (tetrahydrate) | |||
| ⚫ | | Section3 = {{Chembox Structure | ||
| ⚫ | |||
| | Coordination = octahedral | |||
| ⚫ | | Section3 = {{Chembox |
||
| | CrystalStruct = orthrhombic | |||
| | ExternalMSDS = | |||
| | Dipole = 0 ] | |||
| ⚫ | | NFPA-H = |
||
| ⚫ | }} | ||
| ⚫ | | NFPA-R = 0 | ||
| | Section7 = {{Chembox Hazards | |||
| ⚫ | | NFPA-F = |
||
| | ExternalMSDS = | |||
| ⚫ | | FlashPt = | ||
| | EUIndex = Not listed | |||
| | Autoignition = | |||
| | MainHazards = Toxic | |||
| ⚫ | | LD50 = |
||
| | RPhrases = | |||
| ⚫ | |||
| | SPhrases = | |||
| ⚫ | | NFPA-H = 2 | ||
| ⚫ | | NFPA-F = 1 | ||
| ⚫ | | NFPA-R = 0 | ||
| | NFPA-O = | |||
| ⚫ | | FlashPt = 210 °C | ||
| ⚫ | | LD50 = 150 mg/kg (oral, mouse) <br> 230 mg/kg (oral, rat) | ||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | OtherCations = ]<br/>] | |||
| | OtherCpds = ]<br/>] | |||
| }} | |||
| }} | }} | ||
Revision as of 12:45, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 473097283 of page Chromium_hexacarbonyl with values updated to verified values. |
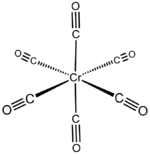
| |
| Names | |
|---|---|
| IUPAC name Hexacarbonylchromium | |
| Other names Chromium carbonyl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| RTECS number |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6CrO6 |
| Molar mass | 220.057 g/mol |
| Appearance | colorless crystals |
| Density | 1.77 g/cm, solid |
| Melting point | 150 °C |
| Boiling point | 210 °C (decomp) |
| Solubility in water | insoluble |
| Solubility | soluble in ether, chloroform, tetrahydropyran (THP), methylene chloride |
| Structure | |
| Crystal structure | orthrhombic |
| Coordination geometry | octahedral |
| Dipole moment | 0 D |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Toxic |
| NFPA 704 (fire diamond) |
 |
| Flash point | 210 °C |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 150 mg/kg (oral, mouse) 230 mg/kg (oral, rat) |
| Related compounds | |
| Other cations | Molybdenum hexacarbonyl Tungsten hexacarbonyl |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chemical compound