| Revision as of 17:43, 16 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 444142387 of page 3,4-Dihydroxymandelic_acid for the Chem/Drugbox validation project (updated: 'CASNo').← Previous edit |
Revision as of 17:43, 16 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 455339255 of page 3,4-Dihydroxyphenylacetaldehyde for the Chem/Drugbox validation project (updated: 'CASNo').Next edit → |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{chembox |
|
{{Chembox |
|
| verifiedrevid = 443317632 |
|
| verifiedrevid = 444177457 |
|
|
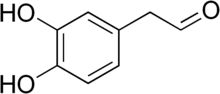
| ImageFile = Dihydroxyphenylacetaldehyde.png |
|
|ImageFile=3,4-Dihydroxymandelic acid.svg |
|
|
|
| ImageFile_Ref = {{chemboximage|correct|??}} |
|
|ImageSize= |
|
|
|
| ImageName = Kekulé, skeletal formula of 3,4-dihydroxyphenylacetaldehyde |
|
|IUPACName=2-(3,4-dihydroxyphenyl)-2-hydroxyacetic acid |
|
|
|
| SystematicName = 2-(3,4-Dihydroxyphenyl)acetaldehyde<ref>{{Cite web|title = 3,4-dihydroxyphenylacetaldehyde - Compound Summary|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=119219|work = PubChem Compound|publisher = National Center for Biotechnology Information|accessdate = 13 October 2011|location = USA|date = 24 June 2005|at = Identification and Related Records}}</ref> |
|
|OtherNames= |
|
|
|Section1={{Chembox Identifiers |
|
| Section1 = {{Chembox Identifiers |
|
|
| Abbreviations = DOPAL |
|
⚫ |
| CASNo = <!-- blanked - oldvalue: 5707-55-1 --> |
|
⚫ |
| PubChem = 119219 |
|
|
| PubChem_Ref = {{Pubchemcite|correct|Pubchem}} |
|
|
| ChemSpiderID = 106504 |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 77371 |
|
| KEGG = C04043 |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
|
| MeSHName = 3,4-dihydroxyphenylacetaldehyde |
|
| KEGG = C05580 |
|
|
|
| ChEBI = 27978 |
| ⚫ |
| InChI = 1/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13) |
|
|
| InChIKey = RGHMISIYKIHAJW-UHFFFAOYAB |
|
| ⚫ |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| ⚫ |
| StdInChI = 1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13) |
|
| ⚫ |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| ⚫ |
| StdInChIKey = RGHMISIYKIHAJW-UHFFFAOYSA-N |
|
| ⚫ |
| CASNo = <!-- blanked - oldvalue: 775-01-9 --> |
|
| ⚫ |
| PubChem=85782 |
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
| ChEBI = 27637 |
|
| 3DMet = B00668 |
|
| SMILES = O=C(O)C(O)c1cc(O)c(O)cc1 |
|
| SMILES = Oc1ccc(CC=O)cc1O |
|
|
| SMILES1 = OC1=CC=C(CC=O)C=C1O |
|
| MeSHName=3,4-dihydroxymandelic+acid |
|
|
⚫ |
| StdInChI = 1S/C8H8O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,4-5,10-11H,3H2 |
| ⚫ |
}} |
|
|
⚫ |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| ⚫ |
|Section2={{Chembox Properties |
|
|
⚫ |
| InChI = 1/C8H8O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,4-5,10-11H,3H2 |
|
| Formula=C<sub>8</sub>H<sub>8</sub>O<sub>5</sub> |
|
|
⚫ |
| StdInChIKey = IADQVXRMSNIUEL-UHFFFAOYSA-N |
|
| MolarMass=184.14612 |
|
|
⚫ |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| Appearance= |
|
|
|
| InChIKey = IADQVXRMSNIUEL-UHFFFAOYAV |
|
| Density= |
|
|
⚫ |
}} |
|
| MeltingPt= |
|
|
⚫ |
| Section2 = {{Chembox Properties |
|
| BoilingPt= |
|
|
| Solubility= |
|
| C = 8 |
|
}} |
|
| H = 8 |
|
|
| O = 3 |
| ⚫ |
|Section3={{Chembox Hazards |
|
|
|
| ExactMass = 152.047344122 g mol<sup>-1</sup> |
|
| MainHazards= |
|
|
⚫ |
}} |
|
| FlashPt= |
|
|
⚫ |
| Section3 = {{Chembox Related |
|
| Autoignition= |
|
|
|
| Function = 2-phenyl aldehydes |
| ⚫ |
}} |
|
|
|
| OtherFunctn = ]<br /> |
|
|
] |
|
|
}} |
|
}} |
|
}} |