| Revision as of 04:37, 17 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 477308048 of page Indium(III)_chloride for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 04:37, 17 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 477307709 of page Indium_phosphide for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 414433478 | ||
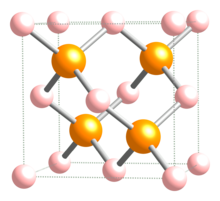
| | ImageFile = InPcrystal.jpg | |||
| ⚫ | | |
||
| | |
| ImageFile2 = Boron-phosphide-unit-cell-1963-CM-3D-balls.png | ||
| | ImageSize = | |||
| | ImageName = Indium(III) chloride | |||
| | ImageName = | |||
| | OtherNames = Indium chloride | |||
| | IUPACName = | |||
| ⚫ | | OtherNames = Indium(III) phosphide | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = |
| ChemSpiderID = 28914 | ||
| ⚫ | | InChI = 1/In.P/rInP/c1-2 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | |
| SMILES = #P | ||
| | InChIKey = GPXJNWSHGFTCBW-HIYQQWJCAF | |||
| ⚫ | | InChI = 1/ |
||
| | SMILES = Cl(Cl)Cl | |||
| | InChIKey = PSCMQHVBLHHWTO-DFZHHIFOAF | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/ |
| StdInChI = 1S/In.P | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = GPXJNWSHGFTCBW-UHFFFAOYSA-N | ||
| | CASNo = |
| CASNo = 22398-80-7 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | PubChem = |
| PubChem = 31170 | ||
| }} | |||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = InP | ||
| | |
| MolarMass = 145.792 g/mol | ||
| | |
| Appearance = black ] crystals | ||
| | |
| Density = 4.81 g/cm<sup>3</sup>, solid | ||
| | MeltingPtC = 1062 | |||
| | Solubility = soluble, exothermic | |||
| ⚫ | | BoilingPt = | ||
| | Solvent = other solvents | |||
| | Solubility = slightly soluble in ]s<ref name="hand"> | |||
| | SolubleOther = ] | |||
| {{Citation | |||
| | MeltingPt = 586 °C | |||
| |last = Lide | |||
| ⚫ | | |
||
| |first = David R. | |||
| ⚫ | |||
| |year = 1998 | |||
| |title = Handbook of Chemistry and Physics | |||
| |edition = 87 | |||
| |publication-place = Boca Raton, FL | |||
| |publisher = CRC Press | |||
| |isbn = 0-8493-0594-2 | |||
| |pages = 4–61 | |||
| }}</ref> | |||
| | BandGap = 1.344 eV (300 K; ]) | |||
| | ElectronMobility = 5400 cm<sup>2</sup>/(V·s) (300 K) | |||
| | ThermalConductivity = 0.68 W/(cm·K) (300 K) | |||
| | RefractIndex = 3.1 (infrared); <br/> 3.55 (632.8 nm)<ref>{{Citation|doi=10.1007/BF00626698|title=The refractive index of InP and its oxide measured by multiple-angle incident ellipsometry|year=1993|last1=Sheng Chao|first1=Tien|last2=Lee|first2=Chung Len|last3=Lei|first3=Tan Fu|journal=Journal of Materials Science Letters|volume=12|pages=721|postscript=.|issue=10}}</ref> | |||
| ⚫ | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | Coordination = Tetrahedral | |||
| | CrystalStruct = ], ] | |||
| | CrystalStruct = ] | |||
| | SpaceGroup = C12/m1, No. 12 | |||
| }} | |||
| | Section4 = {{Chembox Thermochemistry | |||
| | DeltaHf = -88.7 ] | |||
| | DeltaHc = | |||
| | Entropy = 59.8 J/(mol·K) | |||
| | HeatCapacity = 45.4 J/(mol·K)<ref name="hand2"> | |||
| {{Citation | |||
| |last = Lide | |||
| |first = David R. | |||
| |year = 1998 | |||
| |title = Handbook of Chemistry and Physics | |||
| |edition = 87 | |||
| |publication-place = Boca Raton, FL | |||
| |publisher = CRC Press | |||
| |isbn = 0-8493-0594-2 | |||
| |pages = 5–20 | |||
| }}</ref> | |||
| ⚫ | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | ExternalMSDS = | |||
| | |
| EUIndex = Not listed | ||
| ⚫ | | |
||
| | MainHazards = Toxic, hydrolysis to ] | |||
| ⚫ | | |
||
| | |
| NFPA-H = | ||
| ⚫ | | NFPA-F = | ||
| ⚫ | |||
| ⚫ | | NFPA-R = | ||
| | NFPA-O = | |||
| | FlashPt = | |||
| ⚫ | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherAnions = ]<br/>]<br/>] | ||
| | |
| OtherCations = ]<br/>] | ||
| | OtherCpds = ]<br/>]<br/>] | |||
| ⚫ | |||
| }} | |||
| }} | }} | ||
Revision as of 04:37, 17 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 477307709 of page Indium_phosphide with values updated to verified values. |

| |
| |
| Names | |
|---|---|
| Other names Indium(III) phosphide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | InP |
| Molar mass | 145.792 g/mol |
| Appearance | black cubic crystals |
| Density | 4.81 g/cm, solid |
| Melting point | 1,062 °C (1,944 °F; 1,335 K) |
| Solubility in water | slightly soluble in acids |
| Band gap | 1.344 eV (300 K; direct) |
| Electron mobility | 5400 cm/(V·s) (300 K) |
| Thermal conductivity | 0.68 W/(cm·K) (300 K) |
| Refractive index (nD) | 3.1 (infrared); 3.55 (632.8 nm) |
| Structure | |
| Crystal structure | Zinc blende |
| Coordination geometry | Tetrahedral |
| Thermochemistry | |
| Heat capacity (C) | 45.4 J/(mol·K) |
| Std molar entropy (S298) |
59.8 J/(mol·K) |
| Std enthalpy of formation (ΔfH298) |
-88.7 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Toxic, hydrolysis to phosphine |
| Related compounds | |
| Other anions | Indium nitride Indium arsenide Indium antimonide |
| Other cations | Aluminium phosphide Gallium phosphide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–61, ISBN 0-8493-0594-2
- Sheng Chao, Tien; Lee, Chung Len; Lei, Tan Fu (1993), "The refractive index of InP and its oxide measured by multiple-angle incident ellipsometry", Journal of Materials Science Letters, 12 (10): 721, doi:10.1007/BF00626698.
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 5–20, ISBN 0-8493-0594-2