| Revision as of 04:37, 17 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 477307709 of page Indium_phosphide for the Chem/Drugbox validation project (updated: '').← Previous edit | Revision as of 04:37, 17 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 477307276 of page Indium_nitride for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 477001014 | ||
| | ImageFile = |
| ImageFile = Wurtzite polyhedra.png | ||
| | ImageFile2 = Boron-phosphide-unit-cell-1963-CM-3D-balls.png | |||
| | ImageSize = | |||
| | ImageName = | | ImageName = | ||
| | IUPACName = | | IUPACName = | ||
| | OtherNames = Indium(III) |
| OtherNames = Indium(III) nitride | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = |
| ChemSpiderID = 105058 | ||
| | InChI = 1/In. |
| InChI = 1/In.N/rInN/c1-2 | ||
| | SMILES = # |
| SMILES = #N | ||
| | InChIKey = |
| InChIKey = NWAIGJYBQQYSPW-QCNKTVRGAR | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/In. |
| StdInChI = 1S/In.N | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = |
| StdInChIKey = NWAIGJYBQQYSPW-UHFFFAOYSA-N | ||
| | CASNo = |
| CASNo = 25617-98-5 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | PubChem = |
| PubChem = 117560 | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = |
| Formula = InN | ||
| | MolarMass = |
| MolarMass = 128.83 g/mol | ||
| | Appearance = black |
| Appearance = black powder | ||
| | Density = |
| Density = 6.81 g/cm<sup>3</sup> | ||
| | Solubility = hydrolysis | |||
| | MeltingPtC = 1062 | |||
| | SolubleOther = | |||
| | Solvent = | |||
| | MeltingPt = 1100 °C | |||
| | BoilingPt = | | BoilingPt = | ||
| | BandGap = 0.65 eV (300 K) | |||
| | Solubility = slightly soluble in ]s<ref name="hand"> | |||
| ⚫ | | ElectronMobility = 3200 cm<sup>2</sup>/(V*s) (300 K) | ||
| {{Citation | |||
| ⚫ | | ThermalConductivity = 0.45 W/(cm*K) (300 K) | ||
| |last = Lide | |||
| | RefractIndex = 2.9 | |||
| |first = David R. | |||
| ⚫ | }} | ||
| |year = 1998 | |||
| |title = Handbook of Chemistry and Physics | |||
| |edition = 87 | |||
| |publication-place = Boca Raton, FL | |||
| |publisher = CRC Press | |||
| |isbn = 0-8493-0594-2 | |||
| |pages = 4–61 | |||
| }}</ref> | |||
| | BandGap = 1.344 eV (300 K; ]) | |||
| ⚫ | | ElectronMobility = |
||
| ⚫ | | ThermalConductivity = 0. |
||
| | RefractIndex = 3.1 (infrared); <br/> 3.55 (632.8 nm)<ref>{{Citation|doi=10.1007/BF00626698|title=The refractive index of InP and its oxide measured by multiple-angle incident ellipsometry|year=1993|last1=Sheng Chao|first1=Tien|last2=Lee|first2=Chung Len|last3=Lei|first3=Tan Fu|journal=Journal of Materials Science Letters|volume=12|pages=721|postscript=.|issue=10}}</ref> | |||
| ⚫ | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | CrystalStruct = ] (hexagonal) | |||
| | SpaceGroup = ''C''<sup>4</sup><sub>6v</sub>-''P''6<sub>3</sub>''mc'' | |||
| | Coordination = Tetrahedral | | Coordination = Tetrahedral | ||
| <!--| LattConst_a = 353.3 pm | |||
| | CrystalStruct = ] | |||
| | LattConst_c = 569.3 pm--> | |||
| }} | |||
| | LattConst_alpha = | |||
| | Section4 = {{Chembox Thermochemistry | |||
| | LattConst_beta = | |||
| | DeltaHf = -88.7 ] | |||
| | |
| LattConst_gamma = | ||
| | Entropy = 59.8 J/(mol·K) | |||
| | HeatCapacity = 45.4 J/(mol·K)<ref name="hand2"> | |||
| {{Citation | |||
| |last = Lide | |||
| |first = David R. | |||
| |year = 1998 | |||
| |title = Handbook of Chemistry and Physics | |||
| |edition = 87 | |||
| |publication-place = Boca Raton, FL | |||
| |publisher = CRC Press | |||
| |isbn = 0-8493-0594-2 | |||
| |pages = 5–20 | |||
| }}</ref> | |||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| | ExternalMSDS = | | ExternalMSDS = | ||
| | EUIndex = Not listed | | EUIndex = Not listed | ||
| | MainHazards = |
| MainHazards = Irritant, hydrolysis to ] | ||
| | NFPA-H = | | NFPA-H = | ||
| | NFPA-F = | | NFPA-F = | ||
| Line 78: | Line 55: | ||
| | NFPA-O = | | NFPA-O = | ||
| | FlashPt = | | FlashPt = | ||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | OtherAnions = ]<br/>]<br/>] | | OtherAnions = ]<br/>]<br/>] | ||
| | OtherCations = ]<br/>] | | OtherCations = ]<br/>]<br/>] | ||
| | OtherCpds = ]<br/>]<br/>] | | OtherCpds = ]<br/>] | ||
| }} | }} | ||
| }} | }} | ||
Revision as of 04:37, 17 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 477307276 of page Indium_nitride with values updated to verified values. |
| |
| Names | |
|---|---|
| Other names Indium(III) nitride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | InN |
| Molar mass | 128.83 g/mol |
| Appearance | black powder |
| Density | 6.81 g/cm |
| Melting point | 1100 °C |
| Solubility in water | hydrolysis |
| Band gap | 0.65 eV (300 K) |
| Electron mobility | 3200 cm/(V*s) (300 K) |
| Thermal conductivity | 0.45 W/(cm*K) (300 K) |
| Refractive index (nD) | 2.9 |
| Structure | |
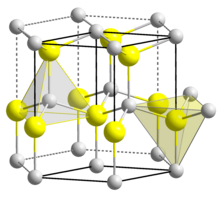
| Crystal structure | Wurtzite (hexagonal) |
| Space group | C6v-P63mc |
| Coordination geometry | Tetrahedral |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant, hydrolysis to ammonia |
| Related compounds | |
| Other anions | Indium phosphide Indium arsenide Indium antimonide |
| Other cations | Boron nitride Aluminium nitride Gallium nitride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |