| Revision as of 09:04, 3 March 2012 edit82.132.236.222 (talk)No edit summary← Previous edit | Revision as of 12:47, 3 March 2012 edit undoEdgar181 (talk | contribs)Extended confirmed users196,325 edits undid recent additions which contained multiple inaccuraciesNext edit → | ||
| Line 2: | Line 2: | ||
| {{refimprove|date=April 2009}} | {{refimprove|date=April 2009}} | ||
| ]s are often found in vapour phase.]] | ]s are often found in vapour phase.]] | ||
| A '''dimer''' is a ] entity consisting of two structurally similar ]s joined by bonds that can be either strong or weak. |
A '''dimer''' is a ] entity consisting of two structurally similar ]s joined by bonds that can be either strong or weak. | ||
| What you have in the diagram below is two cyclopentene molecules which are joined two each other by two single covalent bonds. Structurally this has resulted in the removal of two hydrogens per covalent bond formed (four in total). | |||
| == Organic chemistry == | == Organic chemistry == | ||
Revision as of 12:47, 3 March 2012
For other uses, see Dimer (disambiguation).| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Dimerization" – news · newspapers · books · scholar · JSTOR (April 2009) (Learn how and when to remove this message) |

A dimer is a chemical entity consisting of two structurally similar monomers joined by bonds that can be either strong or weak.
Organic chemistry
Molecular dimers are often formed by the reaction of two identical compounds e.g.: 2A → A-A. In this example, monomer "A" is said to dimerise to give the dimer "A-A". An example is Diaminocarbenes, which dimerise to give tetraaminoethylenes:
- 2 C(NR2)2 → (R2N)2C=C(NR2)2
Acetic acid forms a dimer in the gas phase, the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer.
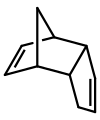
Dicyclopentadiene is an asymmetrical dimer of two cyclopentadiene molecules that have reacted in a Diels-Alder reaction to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers:
- C10H12 → 2 C5H6
The term homodimer is used when the two molecules are identical (e.g. A-A) and heterodimer when they are not (e.g. A-B). The reverse of dimerisation is often called dissociation.
See also
References
- "IUPAC "Gold Book" definition". Retrieved 2009-04-30.