| Revision as of 05:57, 24 September 2011 editAvicBot (talk | contribs)Bots1,227,735 editsm Robot: Fixing double redirect to Walter Reppe← Previous edit | Revision as of 01:45, 21 October 2012 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,550 edits start article, mainly with material from Walter ReppeNext edit → | ||
| Line 1: | Line 1: | ||
| '''Reppe chemistry''' refers to a class of ] reactions that are useful in the synthesis of ]s. Classic Reppe reactions include hydrocarboxylation and esterification of ]s, involving the metal-catalysed reaction with ] and ] or ]s.<ref>El Ali, B.; Alper, H. "Hydrocarboxylation and hydroesterification | |||
| #REDIRECT ] | |||
| reactions catalyzed by transition metal complexes" In Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH:Weinheim, 2004. ISBN: 978-3-527-30613-8</ref> | |||
| ==Reactions involving alknes== | |||
| The hydrocarboxylation of alkenes is a prominent example of Reppe chemistry. In industry, propanoic acid is mainly produced by the hydrocarboxylation of ] using ] as the catalyst:<ref name=Ullmann>{{ Ullmann | author = W. Bertleff; M. Roeper; X. Sava | title = Carbonylation | doi = 10.1002/14356007.a05_217 }}</ref> | |||
| :H<sub>2</sub>C=CH<sub>2</sub> + H<sub>2</sub>O + CO → CH<sub>3</sub>CH<sub>2</sub>CO<sub>2</sub>H | |||
| The hydroesterification is similar, but entails the addition of alcohols instead of water, affording esters. | |||
| ==Reactions involving alknes== | |||
| The high pressure reactions catalysed by heavy metal ]s, especially ], or metal ]s are called '''Reppe Chemistry'''. Reactions can be classified into various classes. The ]ization according to the equation: | |||
| :] | |||
| Preparing ethynyldiols from ]s according to the equation: | |||
| :] | |||
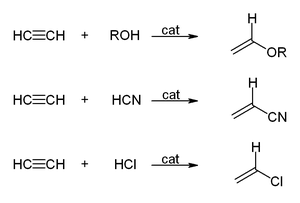
| Reactions with ]: | |||
| :] | |||
| :] | |||
| This simple synthesis was used to prepare ] derivatives for the production of ]. | |||
| ==References== | |||
| <references/> | |||
| ] | |||
Revision as of 01:45, 21 October 2012
Reppe chemistry refers to a class of carbonylation reactions that are useful in the synthesis of organic compounds. Classic Reppe reactions include hydrocarboxylation and esterification of alkenes, involving the metal-catalysed reaction with carbon monoxide and water or alcohols.
Reactions involving alknes
The hydrocarboxylation of alkenes is a prominent example of Reppe chemistry. In industry, propanoic acid is mainly produced by the hydrocarboxylation of ethylene using nickel carbonyl as the catalyst:
- H2C=CH2 + H2O + CO → CH3CH2CO2H
The hydroesterification is similar, but entails the addition of alcohols instead of water, affording esters.
Reactions involving alknes
The high pressure reactions catalysed by heavy metal acetylides, especially copper acetylide, or metal carbonyls are called Reppe Chemistry. Reactions can be classified into various classes. The vinylization according to the equation:
Preparing ethynyldiols from aldehydes according to the equation:
Reactions with carbon monoxide:
This simple synthesis was used to prepare acrylic acid derivatives for the production of acrylic glass.
References
- El Ali, B.; Alper, H. "Hydrocarboxylation and hydroesterification reactions catalyzed by transition metal complexes" In Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH:Weinheim, 2004. ISBN: 978-3-527-30613-8
- W. Bertleff; M. Roeper; X. Sava. "Carbonylation". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_217. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)