| Revision as of 05:46, 3 October 2013 editAnrnusna (talk | contribs)3,532 editsm →Pharmacological effects: journal name, replaced: Nature Struct Biol → Nature Structural & Molecular Biology using AWB← Previous edit | Revision as of 17:34, 26 December 2013 edit undoQuackGuru (talk | contribs)Extended confirmed users79,978 edits →Pharmacological effects: Huperzine A is extracted from huperzia serrata.Next edit → | ||
| Line 64: | Line 64: | ||
| ==Pharmacological effects== | ==Pharmacological effects== | ||
| Huperzine A is an ] inhibititor<ref name="Alzheimer 1996">{{cite journal|pmid=19221692|year=2009|last1=Wang|first1=BS|last2=Wang|first2=H|last3=Wei|first3=ZH|last4=Song|first4=YY|last5=Zhang|first5=L|last6=Chen|first6=HZ|title=Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis.|volume=116|issue=4|pages=457–65|doi=10.1007/s00702-009-0189-x|journal=Journal of neural transmission (Vienna, Austria : 1996)}}</ref> and ] antagonist.<ref>{{cite journal|pmid=18588864|year=2008|last1=Coleman|first1=BR|last2=Ratcliffe|first2=RH|last3=Oguntayo|first3=SA|last4=Shi|first4=X|last5=Doctor|first5=BP|last6=Gordon|first6=RK|last7=Nambiar|first7=MP|title=+-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats.|volume=175|issue=1-3|pages=387–95|doi=10.1016/j.cbi.2008.05.023|journal=Chemico-biological interactions}}</ref> | Huperzine A is an ] inhibititor<ref name="Alzheimer 1996">{{cite journal|pmid=19221692|year=2009|last1=Wang|first1=BS|last2=Wang|first2=H|last3=Wei|first3=ZH|last4=Song|first4=YY|last5=Zhang|first5=L|last6=Chen|first6=HZ|title=Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis.|volume=116|issue=4|pages=457–65|doi=10.1007/s00702-009-0189-x|journal=Journal of neural transmission (Vienna, Austria : 1996)}}</ref> and ] antagonist.<ref>{{cite journal|pmid=18588864|year=2008|last1=Coleman|first1=BR|last2=Ratcliffe|first2=RH|last3=Oguntayo|first3=SA|last4=Shi|first4=X|last5=Doctor|first5=BP|last6=Gordon|first6=RK|last7=Nambiar|first7=MP|title=+-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats.|volume=175|issue=1-3|pages=387–95|doi=10.1016/j.cbi.2008.05.023|journal=Chemico-biological interactions}}</ref> Huperzine A is extracted from ].<ref>{{cite journal|author = Zangara, A|title = The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease|journal = Pharmacol Biochem Behav.|year = 2003|volume = 75|issue = 3|pages = 675–86|doi = 10.1016/S0091-3057(03)00111-4|pmid = 12895686}}</ref> | ||
| Huperzine A has also attracted the attention of US medical science. It is currently being investigated as a possible treatment for diseases characterized by neurodegeneration – particularly ].<ref>{{cite journal|author = Zangara, A|title = The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease|journal = Pharmacol Biochem Behav.|year = 2003|volume = 75|issue = 3|pages = 675–86|doi = 10.1016/S0091-3057(03)00111-4|pmid = 12895686}}</ref><ref name=r1>{{cite journal|author = Bai, D. L.; Tang, X. C.; He, X. C.|title = Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease|pmid=10637369|journal = Current Medicinal Chemistry|year = 2000|volume = 7 |issue= 3|pages = 355–374}}</ref> It has been found to be an ] of the enzyme ].<ref>{{cite journal|author = Tang, X. C.; He, X. C.; Bai, D. L.|title = Huperzine A: a novel acetylcholinesterase inhibitor|journal = Drugs of the Future|year = 1999|volume = 24|issue = 6|pages = 647–663|doi = 10.1358/dof.1999.024.06.545143}}</ref> The structure of the complex of huperzine A with acetylcholinesterase has been determined<ref>{{cite journal|pmid= 8989325|year=1997|last1= Raves |first1=ML|last2=Harel|first2=M|last3=Pang|first3=Y-P|last4=Silman|first4=I|last5=Kozikowski|first5=AP|last6=Sussman|first6=JL|title=3D structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A.|volume=4|issue=1|pages=57–63|journal=Nature Structural & Molecular Biology}}</ref> by ] (PDB code: ; ). This is the same ] of pharmaceutical drugs such as ] and ] used to treat Alzheimer's disease. Huperzine A is also a ] ] which may either reduce or increase glutamate induced damage in brain and increase ] levels in rats.<ref>{{cite journal|author = Tang, L., Wang, R., Tang, X.|title = Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells|journal = Acta Pharmacologica Sinica|year = 2005|volume = 26|pages = 673–678|doi = 10.1111/j.1745-7254.2005.00130.x|pmid = 15916732|issue = 6}}</ref> | Huperzine A has also attracted the attention of US medical science. It is currently being investigated as a possible treatment for diseases characterized by neurodegeneration – particularly ].<ref>{{cite journal|author = Zangara, A|title = The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease|journal = Pharmacol Biochem Behav.|year = 2003|volume = 75|issue = 3|pages = 675–86|doi = 10.1016/S0091-3057(03)00111-4|pmid = 12895686}}</ref><ref name=r1>{{cite journal|author = Bai, D. L.; Tang, X. C.; He, X. C.|title = Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease|pmid=10637369|journal = Current Medicinal Chemistry|year = 2000|volume = 7 |issue= 3|pages = 355–374}}</ref> It has been found to be an ] of the enzyme ].<ref>{{cite journal|author = Tang, X. C.; He, X. C.; Bai, D. L.|title = Huperzine A: a novel acetylcholinesterase inhibitor|journal = Drugs of the Future|year = 1999|volume = 24|issue = 6|pages = 647–663|doi = 10.1358/dof.1999.024.06.545143}}</ref> The structure of the complex of huperzine A with acetylcholinesterase has been determined<ref>{{cite journal|pmid= 8989325|year=1997|last1= Raves |first1=ML|last2=Harel|first2=M|last3=Pang|first3=Y-P|last4=Silman|first4=I|last5=Kozikowski|first5=AP|last6=Sussman|first6=JL|title=3D structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A.|volume=4|issue=1|pages=57–63|journal=Nature Structural & Molecular Biology}}</ref> by ] (PDB code: ; ). This is the same ] of pharmaceutical drugs such as ] and ] used to treat Alzheimer's disease. Huperzine A is also a ] ] which may either reduce or increase glutamate induced damage in brain and increase ] levels in rats.<ref>{{cite journal|author = Tang, L., Wang, R., Tang, X.|title = Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells|journal = Acta Pharmacologica Sinica|year = 2005|volume = 26|pages = 673–678|doi = 10.1111/j.1745-7254.2005.00130.x|pmid = 15916732|issue = 6}}</ref> | ||
Revision as of 17:34, 26 December 2013
| |

| |
| Names | |
|---|---|
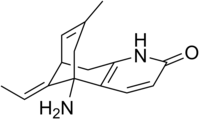
| IUPAC name (1R,9S,13E)-1-Amino-13-ethylidene-11-methyl-6-azatricyclotrideca-2(7),3,10-trien-5-one | |
| Other names HupA | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.132.430 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H18N2O |
| Molar mass | 242.32 g/mol |
| Melting point | 217–219 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata. and in varying quantities in other Huperzia species, including H. elmeri, H. carinat, and H. aqualupian.
Huperzine A is also an acetylcholinesterase inhibitor, which has a mechanism of action similar to donepezil, rivastigmine, and galantamine. A pro-drug form of huperzine A (ZT-1) is under development as a treatment for Alzheimer's disease.
In the US, huperzine A is sold as a dietary supplement for memory support. The botanical has been used in China for centuries for the treatment of swelling, fever and blood disorders. Clinical trials in China have shown it to be effective in improving cognitive performance in patients with Alzheimer's disease and enhancing memory in adolescents complaining of memory inadequacy.
Pharmacological effects
Huperzine A is an acetylcholinesterase inhibititor and NMDA receptor antagonist. Huperzine A is extracted from huperzia serrata.
Huperzine A has also attracted the attention of US medical science. It is currently being investigated as a possible treatment for diseases characterized by neurodegeneration – particularly Alzheimer's disease. It has been found to be an inhibitor of the enzyme acetylcholinesterase. The structure of the complex of huperzine A with acetylcholinesterase has been determined by X-ray crystallography (PDB code: 1VOT; see the 3D structure). This is the same mechanism of action of pharmaceutical drugs such as galantamine and donepezil used to treat Alzheimer's disease. Huperzine A is also a NMDA receptor antagonist which may either reduce or increase glutamate induced damage in brain and increase nerve growth factor levels in rats.
Clinical trials in China have shown that huperzine A is comparably effective to similar drugs on the market, and may even be safer in terms of side effects. The National Institute on Aging has completed a Phase II clinical trial to evaluate the safety and efficacy of huperzine A in the treatment of Alzheimer's disease in a randomized controlled trial of its effect on cognitive function. In 2008, the National Institute on Aging conducted the first controlled trial outside of China evaluating the efficacy and toxicity of huperzine A to improve cognitive function in patients with AD. In this multi-center, double-blind, placebo-controlled Phase II trial, 210 participants with mild to moderate AD received either 200 mcg of huperzine A, 400 mcg of huperzine A, or placebo twice daily for 16 weeks. While no statistical difference in cognitive scores was noted in patients in the lower dose huperzine A group compared to placebo, the higher dose (400 mcg) of huperzine A led to improved cognition and activities of daily living. However, no significant changes were noted in any of the three groups in overall change in disease or in psychiatric ratings according to the AD Assessment Scale-Cognitive (ADAS-Cog) scale. Huperzine A was safe and well tolerated in the study. (13)
The same year, a Cochrane Database review examined studies evaluating the efficacy and safety of huperzine A in the treatment of AD. The review included six randomized, controlled trials involving 454 patients. The conclusion was that the currently available evidence is insufficient to assess the potential for huperzine A in the treatment of MCI. Randomised double-blind placebo-controlled trials are needed.
Possible side effects include breathing problems, tightness in the throat or chest, chest pain, skin hives, rash, itchy or swollen skin, upset stomach, diarrhea, vomiting, hyperactivity and insomnia. Most adverse events were cholinergic in nature and no serious adverse events occurred. Huperzine A is a well-tolerated drug.
Synthesis
Two scalable and efficient total syntheses of huperzine A have been reported.
See also
References
- Kozikowski, Alan P.; Tueckmantel, Werner (1999). "Chemistry, Pharmacology, and Clinical Efficacy of the Chinese Nootropic Agent Huperzine A". Accounts of Chemical Research. 32 (8): 641–650. doi:10.1021/ar9800892.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lim WH, Goodger JQ, Field AR, Holtum JA, Woodrow IE "Huperzine alkaloids from Australasian and southeast Asian Huperzia". Pharm Biol. 2010 Sep;48(9):1073-8
- P. Scalfaro, V. Nicolas, M.P. Simonin, S. Charbon, M. McCormick, F. Heimgartner. The sustained release of the acetylcholinesterase inhibitor ZT-1 confers the potential for a more efficient neuroprotection in rats. Neurobiology of Aging Conference in New Orleans, Nov 2003.
- ^ Wang, Bai-Song; Wang, Hao; Wei, Zhao-hui; Song, Yan-yan; Zhang, Lu; Chen, Hong-Zhuan (2009). "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of Neural Transmission. 116 (4): 457. doi:10.1007/s00702-009-0189-x. PMID 19221692.
- Sun, QQ; Xu, SS; Pan, JL; Guo, HM; Cao, WQ (1999). "Huperzine-A capsules enhance memory and learning performance in 34 pairs of matched adolescent students". Zhongguo yao li xue bao = Acta pharmacologica Sinica. 20 (7): 601–3. PMID 10678121.
- ^ Wang, BS; Wang, H; Wei, ZH; Song, YY; Zhang, L; Chen, HZ (2009). "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of neural transmission (Vienna, Austria : 1996). 116 (4): 457–65. doi:10.1007/s00702-009-0189-x. PMID 19221692.
- Coleman, BR; Ratcliffe, RH; Oguntayo, SA; Shi, X; Doctor, BP; Gordon, RK; Nambiar, MP (2008). "+-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats". Chemico-biological interactions. 175 (1–3): 387–95. doi:10.1016/j.cbi.2008.05.023. PMID 18588864.
- Zangara, A (2003). "The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease". Pharmacol Biochem Behav. 75 (3): 675–86. doi:10.1016/S0091-3057(03)00111-4. PMID 12895686.
- Zangara, A (2003). "The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease". Pharmacol Biochem Behav. 75 (3): 675–86. doi:10.1016/S0091-3057(03)00111-4. PMID 12895686.
- ^ Bai, D. L.; Tang, X. C.; He, X. C. (2000). "Huperzine A, a potential therapeutic agent for treatment of Alzheimer's disease". Current Medicinal Chemistry. 7 (3): 355–374. PMID 10637369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tang, X. C.; He, X. C.; Bai, D. L. (1999). "Huperzine A: a novel acetylcholinesterase inhibitor". Drugs of the Future. 24 (6): 647–663. doi:10.1358/dof.1999.024.06.545143.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Raves, ML; Harel, M; Pang, Y-P; Silman, I; Kozikowski, AP; Sussman, JL (1997). "3D structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A.". Nature Structural & Molecular Biology. 4 (1): 57–63. PMID 8989325.
- Tang, L., Wang, R., Tang, X. (2005). "Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells". Acta Pharmacologica Sinica. 26 (6): 673–678. doi:10.1111/j.1745-7254.2005.00130.x. PMID 15916732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Huperzine A, Alzheimer Research Forum
- Huperzine A for mild cognitive impairment.Yue J, et al. Cochrane Database Syst Rev. 2012 Dec 12. Link http://www.ncbi.nlm.nih.gov/pubmed/23235666
- Tun, M.K.M.; Wüstmann, D-J.; Herzon, S.B. (2011). "A robust and scalable synthesis of the potent neuroprotective agent (−)-huperzine A
". Chemical Science. 2: 2251–2253. doi:10.1039/C1SC00455G.
{{cite journal}}: line feed character in|title=at position 84 (help) - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/op200360b, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/op200360binstead.
| Psychoanaleptics: Anti-dementia agents (ATC code N06D and others) | |
|---|---|
| AChE inhibitor medications | |
| Other medications | |
| Experimental BACE inhibitors | |
| Glutamate receptor modulators | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||