| Revision as of 19:57, 1 January 2014 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,725 edits tidy up wording and redlinks← Previous edit | Revision as of 17:44, 4 January 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,725 edits table of diphosphates, this article is also visited about 10,000x/moNext edit → | ||
| Line 40: | Line 40: | ||
| |} | |} | ||
| In addition to these phosphates, sodium forms a number of useful salts with ]s |
In addition to these phosphates, sodium forms a number of useful salts with ]s (called diphosphates): | ||
| {| class="wikitable" | |||
| |- | |||
| ! name | |||
| ! formula | |||
| |- | |||
| | monosodium diphosphate (anhydrous) ||NaH<sub>3</sub>P<sub>2</sub>O<sub>7</sub> | |||
| |- | |||
| | ] (anhydrous) |Na<sub>2</sub>H<sub>2</sub>P<sub>2</sub>O<sub>7</sub> | |||
| |- | |||
| | ] (hexahydrate) ||Na<sub>2</sub>H<sub>2</sub>P<sub>2</sub>O<sub>7</sub>(H<sub>2</sub>O)<sub>6</sub> | |||
| |- | |||
| | trisodium diphosphate (anhydrous) ||Na<sub>3</sub>HP<sub>2</sub>O<sub>7</sub> | |||
| |- | |||
| | trisodium diphosphate (hydrate) ||Na<sub>3</sub>HP<sub>2</sub>O<sub>7</sub>(H<sub>2</sub>O) | |||
| |- | |||
| | trisodium diphosphate nonahydrate ||Na<sub>3</sub>HP<sub>2</sub>O<sub>7</sub>(H<sub>2</sub>O)<sub>9</sub> | |||
| |- | |||
| | ] (anhydrous) |Na<sub>4</sub>P<sub>2</sub>O<sub>7</sub> | |||
| |- | |||
| | ] (decahydrate) ||Na<sub>4</sub>P<sub>2</sub>O<sub>7</sub>(H<sub>2</sub>O)<sub>10</sub> | |||
| |- | |||
| |} | |||
| triphosphates (linear and cyclic), and polyphosphates. | |||
| ==Applications== | ==Applications== | ||
Revision as of 17:44, 4 January 2014

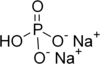

Sodium phosphate is a generic term for a variety of salts of Na and phosphate (PO4), hydrogen phosphate (HPO4), and dihydrogenphosphate (H2PO4). Known compounds are shown in the table. Usually the hydrated salts are more common than the anhydrous forms.
| name | formula |
|---|---|
| monosodium phosphate (anhydrous) | NaH2PO4 |
| monosodium phosphate (monohydrate) | NaH2PO4(H2O) |
| monosodium phosphate (dihydrate) | NaH2PO4(H2O)2 |
| monosodium phosphate (anhydrous) | HNa2PO4 |
| disodium phosphate (dihydrate) | HNa2PO4(H2O)2 |
| disodium phosphate (heptahydrate) | HNa2PO4(H2O)7 |
| disodium phosphate (octahydrate) | HNa2PO4(H2O)8 |
| disodium phosphate (dodecahydrate) | HNa2PO4(H2O)12 |
| trisodium phosphate (anhydrous, hexagonal) | Na3PO4 |
| trisodium phosphate (anhydrous, cubic) | Na3PO4 |
| trisodium phosphate (hemihydrate) | Na3PO4(H2O)0.5 |
| trisodium phosphate (hexahydrate) | Na3PO4(H2O)6 |
| trisodium phosphate (octahydrate) | Na3PO4(H2O)8 |
| trisodium phosphate (dodecahydrate) | Na3PO4(H2O)12 |
In addition to these phosphates, sodium forms a number of useful salts with pyrophosphates (called diphosphates):
| name | formula |
|---|---|
| monosodium diphosphate (anhydrous) | NaH3P2O7 |
| disodium diphosphate (anhydrous) |Na2H2P2O7 | |
| disodium diphosphate (hexahydrate) | Na2H2P2O7(H2O)6 |
| trisodium diphosphate (anhydrous) | Na3HP2O7 |
| trisodium diphosphate (hydrate) | Na3HP2O7(H2O) |
| trisodium diphosphate nonahydrate | Na3HP2O7(H2O)9 |
| tetrasodium phosphate (anhydrous) |Na4P2O7 | |
| tetrasodium diphosphate (decahydrate) | Na4P2O7(H2O)10 |
triphosphates (linear and cyclic), and polyphosphates.
Applications
Sodium phosphates have many applications in the food industry and for water treatment. For example, sodium phosphates are often used as meat preservatives, as an alternative to sodium nitrite. This is common in canned meats.
Safety
Sodium phosphates are popular in commerce in part because they are inexpensive and nontoxic.
Oral sodium phosphates for bowel preparation for colonoscopy may in some individuals carry a risk of kidney injury under the form of phosphate nephropathy. There are several oral phosphate formulations which are prepared extemporaneously. Oral phosphate prep drugs have been withdrawn in the United States, although evidence of causality is equivocal. Since safe and effective replacements for phosphate purgatives are available, several medical authorities have recommended general disuse of oral phosphates.
References
- Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in Ullmann’s Encyclopedia of Industrial Chemistry 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_465.pub3
-
Markowitz, GS; Perazella, MA (Aug 12), "Acute Phosphate Nephropathy", Kidney Int., vol. 76, no. 10, pp. 1027–34, doi:10.1038/ki.2009.308, PMID 19675530
{{citation}}: Check date values in:|date=and|year=/|date=mismatch (help) -
Mackey, AC; Green, L; Amand, KS; Avigan, M (2009), "Sodium phosphate tablets and acute Phosphate Nephropathy", Am J Gastroenterol, vol. 104, no. 8 (published Aug), pp. 1903–6, doi:10.1038/ajg.2009.342, PMID 19661931
{{citation}}: Check date values in:|publication-date=(help)
External links
- Bell, Russel N (1973), "SODIUM ALUMINUM PHOSPHATE CHEESE EMULSIFYING AGENT", US Patent 3726960 (published April)
{{citation}}: Check date values in:|publication-date=(help) - Lien, YH (2008), "Is bowel preparation before colonoscopy a risky business for the kidney?", Nature Clinical Practice Nephrology, 4 (11): 606–14, doi:10.1038/ncpneph0939, PMID 18797448.
| Sodium compounds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic | |||||||||||||||