| Revision as of 18:13, 31 July 2006 view sourceTrevor MacInnis (talk | contribs)Extended confirmed users74,493 editsm Reverted edits by 69.27.229.10 (talk) to last version by 66.173.87.126← Previous edit | Revision as of 03:27, 1 August 2006 view source 67.15.217.15 (talk)No edit summaryNext edit → | ||
| Line 14: | Line 14: | ||
| ICD9 = {{ICD9|042}}-{{ICD9|044}} | | ICD9 = {{ICD9|042}}-{{ICD9|044}} | | ||
| }} | }} | ||
| '''Human immunodeficiency virus''' (commonly known as '''HIV''', and formerly known as '''HTLV-III''' and '''lymphadenopathy-associated virus''') is a ] that is the cause of the disease known as ] (''Acquired Immunodeficiency Syndrome''), a syndrome where the immune system begins to fail, leading to many life-threatening opportunistic infections. | '''Human immunodeficiency virus''' (commonly known as '''HIV''', and formerly known as '''HTLV-III''' and '''lymphadenopathy-associated virus''') is a ] that is the cause of the disease known as ] (''Acquired Immunodeficiency Syndrome''), a syndrome where the immune system begins to fail, leading to many life-threatening opportunistic infections. HIV is commonly spread through anal intercourse, a popular activity for homosexual men. | ||
| HIV primarily infects vital components of the ] ] such as ], ] and ]. It also directly and indirectly destroys CD4<SUP>+</SUP> T cells. As CD4<SUP>+</SUP> T cells are required for the proper functioning of the immune system, when enough CD4<SUP>+</SUP> T cells have been destroyed by HIV, the immune system functions poorly, leading to ]. HIV also directly attacks ]s such as the ]s, ] and ], leading to ], ], ] and ]. Many of the problems faced by people infected with HIV result from failure of the immune system to protect from ]s and ]s. | HIV primarily infects vital components of the ] ] such as ], ] and ]. It also directly and indirectly destroys CD4<SUP>+</SUP> T cells. As CD4<SUP>+</SUP> T cells are required for the proper functioning of the immune system, when enough CD4<SUP>+</SUP> T cells have been destroyed by HIV, the immune system functions poorly, leading to ]. HIV also directly attacks ]s such as the ]s, ] and ], leading to ], ], ] and ]. Many of the problems faced by people infected with HIV result from failure of the immune system to protect from ]s and ]s. | ||
Revision as of 03:27, 1 August 2006
Template:Taxobox begin Template:Taxobox image Template:Taxobox begin placement virus Template:Taxobox group vi entry Template:Taxobox familia entry Template:Taxobox genus entry Template:Taxobox species entry Template:Taxobox species entry Template:Taxobox end placement Template:Taxobox end
Medical condition| HIV | |
|---|---|
| Frequency | 0.6—0.9% |
Human immunodeficiency virus (commonly known as HIV, and formerly known as HTLV-III and lymphadenopathy-associated virus) is a retrovirus that is the cause of the disease known as AIDS (Acquired Immunodeficiency Syndrome), a syndrome where the immune system begins to fail, leading to many life-threatening opportunistic infections. HIV is commonly spread through anal intercourse, a popular activity for homosexual men.
HIV primarily infects vital components of the human immune system such as CD4 T cells, macrophages and dendritic cells. It also directly and indirectly destroys CD4 T cells. As CD4 T cells are required for the proper functioning of the immune system, when enough CD4 T cells have been destroyed by HIV, the immune system functions poorly, leading to AIDS. HIV also directly attacks organs such as the kidneys, heart and brain, leading to acute renal failure, cardiomyopathy, dementia and encephalopathy. Many of the problems faced by people infected with HIV result from failure of the immune system to protect from opportunistic infections and cancers.
HIV is transmitted through direct contact of a mucous membrane with a bodily fluid containing HIV, such as blood, semen, vaginal fluid, preseminal fluid or breast milk. This transmission can come in the form of: penetrative (anal or vaginal) sex; oral sex; blood transfusion; contaminated needles; exchange between mother and infant during pregnancy, childbirth, or breastfeeding; or other exposure to one of the above bodily fluids.
Infection in humans is now pandemic. As of January 2006, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) estimate that AIDS has killed more than 25 million people since it was first recognized on December 1, 1981, making it one of the most destructive pandemics in recorded history. In 2005 alone, AIDS claimed an estimated 2.4—3.3 million lives, of which more than 570,000 were children. A third of these deaths are occurring in sub-Saharan Africa, retarding economic growth by destroying human capital. Current estimates state that HIV is set to infect 90 million people in Africa, resulting in a minimum estimate of 18 million orphans. Antiretroviral treatment reduces both the mortality and the morbidity of HIV infection, but routine access to antiretroviral medication is not available in all countries.
Origin and discovery
Main article: AIDS originThe AIDS epidemic was discovered June 5, 1981, when the U.S. Centers for Disease Control and Prevention reported a cluster of Pneumocystis carinii pneumonia (now classified as Pneumocystis jiroveci pneumonia) in five homosexual men in Los Angeles. Originally dubbed GRID, or Gay-Related Immune Deficiency, health authorities soon realized that nearly half of the people identified with the syndrome were not homosexual men. In 1982, the CDC introduced the term AIDS to describe the newly recognized syndrome, though it was still casually referred to as GRID.
In 1983, scientists led by Luc Montagnier at the Pasteur Institute in France first discovered the virus that causes AIDS. They called it lymphadenopathy-associated virus (LAV). A year later a team led by Robert Gallo of the United States confirmed the discovery of the virus, but they renamed it human T lymphotropic virus type III (HTLV-III). The dual discovery led to considerable scientific fallout, and it was not until President Mitterrand of France and President Reagan of the USA met that the major issues were ironed out. In 1986, both the French and the US names for the virus itself were dropped in favour of the new term, human immunodeficiency virus (HIV).
HIV was classified as a member of the genus lentivirus, part of the family of retroviridae. Lentiviruses have many common morphologies and biological properties. Many species are infected by lentiviruses, which are characteristically responsible for long duration illnesses associated with a long period of incubation. Lentiviruses are transmitted as single-stranded, positive-sense, enveloped RNA viruses. Upon infection of the target cell, the viral RNA genome is converted to double-stranded DNA by a virally encoded reverse transcriptase which is present in the virus particle. This viral DNA is then integrated into the cellular DNA by a virally encoded integrase so that replication using cellular machinery may take place. Once the virus enters the cell, two pathways are possible: either the virus becomes latent and the infected cell continues to function, or the virus becomes active and replicates, and a large number of virus particles are liberated which can infect other cells.
Two species of HIV infect humans: HIV-1 and HIV-2. HIV-1 is hypothesized to have originated in southern Cameroon after jumping from wild chimpanzees (Pan troglodytes troglodytes) to humans during the twentieth century. HIV-2 is hypothesized to have originated from the Sooty Mangabey (Cercocebus atys), an Old World monkey of Guinea-Bissau, Gabon, and Cameroon. HIV-1 is more virulent, more easily transmitted and is the cause of the majority of HIV infections globally, while HIV-2 is less easily transmitted and is largely confined to West Africa. HIV-1 is the virus that was initially discovered and termed LAV.
Three of the earliest known instances of HIV-1 infection are as follows:
- A plasma sample taken in 1959 from an adult male living in what is now the Democratic Republic of Congo.
- HIV found in tissue samples from a 15 year old African-American teenager who died in St. Louis in 1969.
- HIV found in tissue samples from a Norwegian sailor who died around 1976.
Although a variety of theories exist explaining the transfer of HIV to humans, there is no widely accepted scientific consensus of any single hypothesis and the topic remains controversial. Freelance journalist Tom Curtis discussed one currently controversial possibility for the origin of HIV/AIDS in a 1992 Rolling Stone magazine article. He put forward what is now known as the OPV AIDS hypothesis, which suggests that AIDS was inadvertently caused in the late 1950s in the Belgian Congo by Hilary Koprowski's research into a polio vaccine. Although subsequently retracted due to libel issues surrounding its claims, the Rolling Stone article motivated another freelance journalist, Edward Hooper, to probe more deeply into this subject. Hooper's research resulted in his publishing a 1999 book, The River, in which he alleged that an experimental oral polio vaccine prepared using chimpanzee kidney tissue was the route through which simian immunodeficiency virus (SIV) crossed into humans to become HIV, thus starting the human AIDS pandemic.
Transmission
- For more details on this topic, see AIDS transmission and prevention
| Estimated per act risk for acquisition of HIV by exposure route | ||||
|---|---|---|---|---|
| Exposure Route | Estimated infections per 10,000 exposures to an infected source | |||
| Blood Transfusion | 9,000 | |||
| Childbirth | 2,500 | |||
| Needle-sharing injection drug use- | 67 | |||
| Receptive anal intercourse | 50 | |||
| Percutaneous needle stick | 30 | |||
| Receptive penile-vaginal intercourse | 10 | |||
| Insertive anal intercourse | 6.5 | |||
| Insertive penile-vaginal intercourse | 5 | |||
| Receptive fellatio | 1 | |||
| Insertive fellatio | 0.5 | |||
| assuming no condom use | ||||
Since the beginning of the pandemic, three main transmission routes of HIV have been identified:
- Sexual route. The majority of HIV infections are acquired through unprotected sexual relations. Sexual transmission occurs when there is contact between sexual secretions of one partner with the rectal, genital or oral mucous membranes of another.
- Blood or blood product route. This transmission route is particularly important for intravenous drug users, hemophiliacs and recipients of blood transfusions (though most transfusions are checked for HIV) and blood products. It is also of concern for persons receiving medical care in regions where there is prevalent substandard hygiene in the use of injection equipment (e.g. reused needles in Third World settings). Health care workers (nurses, laboratory workers, doctors, etc) are also directly concerned, although more rarely. Also concerned by this route are people who give and receive tattoos, piercings and scarification procedures.
- Mother-to-child transmission (MTCT). The transmission of the virus from the mother to the child can occur in utero during the last weeks of pregnancy and at childbirth. In the absence of treatment, the transmission rate between the mother and child is 25%. However, where treatment is available, combined with the availability of Cesarian section, this has been reduced to 1%. Breast feeding also presents a risk of infection for the baby.
HIV has been found at low concentrations in the saliva, tears and urine of infected individuals, but the risk of transmission by these secretions is negligible. The use of physical barriers such as the latex condom is widely advocated to reduce the sexual transmission of HIV. Current research is clarifying the relationship between male circumcision and HIV in differing social and cultural contexts. UNAIDS believes that it is premature to recommend male circumcision services as part of HIV prevention programs even though male circumcision may lead to a reduction of infection risk in heterosexual men by up to 60%. Moreover, South African medical experts are concerned that the repeated use of unsterilized blades in the ritual circumcision of adolescent boys may be spreading HIV.
Structure and genome
Main article: HIV structure and genomeHIV is different in structure from previously described retroviruses. It is about 120 nm in diameter (120 billionths of a meter; around 60 times smaller than a red blood cell) and roughly spherical.
It is composed of two copies of positive single-stranded RNA enclosed by a conical capsid, which is in turn surrounded by a plasma membrane that is formed from part of the former host-cell membrane. Other enzymes contained within the virion particle include reverse transcriptase, integrase, and protease.
HIV has several major genes coding for structural proteins that are found in all retroviruses, and several nonstructural ("accessory") genes that are unique to it. The gag gene provides the physical infrastructure of the virus; pol provides the basic enzymes by which retroviruses reproduce; the env gene supplies the proteins essential for viral attachment and entry into a target cell. The accessory proteins tat, rev, nef, vif, vpr, and vpu enhance virus production. Although called accessory proteins, tat and rev transactivators are essential for virus replication.
In some strains of HIV, a mutation causes the production of an alternate accessory protein, Tev, from the fusion of tat, rev, and env.
The gp120 and gp41 proteins, both encoded by the env gene form gp160 before cleavage to two separate proteins, enable the virus to attach to and fuse with target cells to initiate the infectious cycle. Both, especially gp120, have been considered as targets of future treatments or vaccines against HIV.
Tropism
The term viral tropism refers to the cell type into which HIV may infect and replicate within. HIV can infect a variety of cells such as CD4 T cells, macrophages, and microglial cells. HIV-1 entry to macrophages and CD4 T cells is mediated not only through interaction of the virion envelope glycoproteins (gp120) with the CD4 molecule on the target cells but also with its chemokine coreceptors.
Macrophage (M-tropic) strains of HIV-1, or non-syncitia-inducing strains (NSI) use the β-chemokine receptor CCR5 for entry and are thus able to replicate in macrophages and CD4 T cells. The normal ligands for this receptor, RANTES, macrophage inflammatory protein (MIP)-1-beta and MIP-1-alpha, are able to suppress HIV-1 infection in vitro. This CCR5 coreceptor is used by almost all primary HIV-1 isolates regardless of viral genetic subtype. Indeed, macrophages play a key role in several critical aspects of HIV disease. They appear to be the first cells infected by HIV and perhaps the very source of HIV production when CD4 cells are markedly depleted in the patient. Macrophages and microglial cells are the cells infected by HIV in the central nervous system. In tonsils and adenoids of HIV-infected patients, macrophages fuse into multinucleated giant cells that produce copious amounts of virus.
T-tropic isolates, or syncitia-inducing (SI) strains replicate in primary CD4 T cells as well as in macrophages and use the α-chemokine receptor, CXCR4, for entry. The α-chemokine, SDF-1, a ligand for CXCR4, suppresses replication of T-tropic HIV-1 isolates. It does this by down regulating the expression of CXCR4 on the surface of these cells. HIV that use only the CCR5 receptor are termed R5, those that only use CXCR4 are termed X4, and those that use both, X4R5. However, the use of coreceptor alone does not explain viral tropism, as not all R5 viruses are able to use CCR5 on macrophages for a productive infection and HIV can also infect a subtype of myeloid dendritic cells, which probably constitute a major reservoir that maintains infection when CD4 T cell numbers have declined to extremely low levels.
Some people are resistant to certain strains of HIV. An example of this is people with the CCR5-Δ32 mutation; these people are resistant to infection with R5 virus as the mutation does not allow HIV to bind to this coreceptor, impeding its ability to infect the target cell.
Heterosexual intercourse is the major mode of HIV transmission. Both X4 and R5 HIV are present in the seminal fluid as free or cell associated particles which are passed from partner to partner where the virions can then infect numerous cellular targets and disseminate into the whole organism. However, a selection process has been shown to lead to a predominant transmission of the R5 virus through this pathway. The mechanism of this selective process is still under investigation, though recent data suggest that spermatozoa may selectively carry R5 HIV as they possess both CCR3 and CCR5 but not CXCR4 on their surface and that genital epithelial cells preferentially sequester X4 virus. In patients infected with subtype B HIV-1, there is often a co-receptor switch in late stage disease and T-tropic variants appear that can infect a variety of T cells via CXCR4. These variants then replicate more aggressively with heightened virulence that facilitates rapid T cell depletion, immune system collapse, and opportunistic infection that marks the advent of AIDS. Thus, during the course of infection, viral adaptation to the use of CXCR4 instead of CCR5 is often seen as a key step in the progression to AIDS. A number of studies with subtype B-infected individuals have determined that between 40 and 50% of AIDS patients can harbour viruses of the SI, and presumably the X4, phenotype.
Replication cycle
 |
 |
Entry to the cell
HIV enters macrophages and CD4 T cells by the adsorption of glycoproteins on its surface to receptors on the target cell followed by fusion of the viral envelope with the cell membrane and the release of the HIV capsid into the cell.
The interactions of the trimeric envelope complex (gp160 spike) and both CD4 and a chemokine receptor (generally either CCR5 or CXCR4 but others are known to interact) on the cell surface. The gp160 spike is composed of three transmembrane glycoproteins (gp41), which anchor the cluster to the virus, and three extracellular glycoproteins (gp120), which contain the binding domains for both CD4 and chemokine receptors. The first step in fusion involves the high-affinity attachment of the CD4 binding domains of gp120 to the N-terminal membrane-distal domains of CD4. Once gp120 is bound with the CD4 protein, the envelope complex undergoes a structural change, exposing the chemokine binding domains of gp120 and allowing them to interact with the target chemokine receptor. This allows for a more stable two-pronged attachment, which leads to the N-terminal fusion peptide gp41 to penetrate the cell membrane. Two heptad repeat sequences of gp41, HR1 and HR2, then interact, resulting in the collapse of the extracellular portion of gp41 forming a hairpin. This hairpin structure brings the virus and cell membranes close together, allowing fusion of the membranes and subsequent entry of the viral capsid.
HIV infects dendritic cells (DCs) by intimate contact of naive CD4 T cells during the course of antigen presentation by a gp120/gp41 independent route using mannose-specific C-type lectin receptors such as DC-SIGN.
Once HIV has bound to the target cell, the HIV RNA and various enzymes, including but not limited to reverse transcriptase, integrase and protease, are injected into the cell.
Replication and transcription
Once the viral capsid has entered the cell, an enzyme called reverse transcriptase liberates the single-stranded (+)RNA from the attached viral proteins and copies it into a negatively sensed viral complementary DNA of 9 kb pairs (cDNA). This process of reverse transcription is extremely error prone and it is during this step that mutations (such as drug resistance) are likely to arise. The reverse transcriptase then makes a complementary DNA strand to form a double-stranded viral DNA intermediate (vDNA). This new vDNA is then transported into the cell nucleus. The integration of the proviral DNA into the host genome is carried out by another viral enzyme called integrase. This is called the latent stage of HIV infection. To actively produce virus, certain cellular transcription factors need to be present, the most important of which is NF-κB (NF kappa B), which is upregulated when the T cell becomes activated. This means that those cells most likely to be killed by HIV are in fact those currently fighting infection.
Initially the integrated provirus is copied to mRNA which is then spliced into smaller chunks. These small chunks produce the regulatory proteins Tat (which encourages new virus production) and Rev. As Rev accumulates it gradually starts to inhibit mRNA splicing. At this stage the structural proteins Gag and Env are produced from the full-length mRNA. Additionally the full-length RNA is actually the virus genome, so it binds to the Gag protein and is packaged into new virus particles.
Interestingly, HIV-1 and HIV-2 appear to package their RNA differently; HIV-1 will bind to any appropriate RNA whereas HIV-2 will preferentially bind to the mRNA which was used to create the Gag protein itself. This may mean that HIV-1 is better able to mutate (HIV-1 infection progresses to AIDS faster than HIV-2 infection and is responsible for the majority of global infections).
Assembly and release
The final step of the viral cycle, assembly of new HIV-1 virions, begins at the plasma membrane of the host cell. The Env polyprotein (gp160) goes through the endoplasmic reticulum and is transported to the Golgi complex where it is cleaved by protease and processed into the two HIV envelope glycoproteins gp41 and gp120. These are transported to the plasma membrane of the host cell where gp41 anchors the gp120 to the membrane of the infected cell. The Gag (p55) and Gag-Pol (p160) polyproteins also associate with the inner surface of the plasma membrane along with the HIV genomic RNA as the forming virion begins to bud from the host cell. Maturation either occurs in the forming bud or in the immature virion after it buds from the host cell. During maturation, HIV proteases (proteinases) cleave the polyproteins into individual functional HIV proteins and enzymes. The various structural components then assemble to produce a mature HIV virion. This step can be inhibited by drugs. The virus is then able to infect another cell.
Genetic variability
 |
One of the major characteristics of HIV is its high genetic variability as a result of its fast replication cycle and the high error rate and recombinogenic properties of reverse transcriptase. This means that different genomic combinations may be generated within an individual who is infected by genetically different HIV strains. Recombination results when a cell is simultaneously infected by two different strains of HIV and one RNA transcript from two different viral strains are encapsidated into the same virion particle. This virion then infects a new cell where it undergoes replication. During this phase, the reverse transcriptase, by jumping back and forth between the two different RNA templates, will generate a newly synthesized retroviral DNA sequence that is a recombinant between the two parental genomes. This recombination is most obvious when it occurs between subtypes.
Three groups of HIV-1 have been identified on the basis of differences in env: M, N, and O. Group M is the most prevalent and is subdivided into eight subtypes (or clades), based on the whole genome, that are each geographically distinct. The most prevalent are subtypes B (found predominantly in North America and Europe), A and D (found predominantly in Africa), and C (found predominantly in Africa and Asia); these subtypes form branches in the phylogenetic tree representing the lineage of the M group of HIV-1. Coinfection with distinct subtypes gives rise to circulating recombinant forms (CRFs).
In 2000, the last year in which an analysis of global subtype prevalence was made, 47.2% of infections worldwide were of subtype C, 26.7% were of subtype A/CRF02_AG, 12.3% were of subtype B, 5.3% were of subtype D, 3.2% were of CRF_AE, and the remaining 5.3% were composed of other subtypes and CRFs. Almost 95% of all HIV research currently taking place is focused on subtype B, while a few laboratories focus on other subtypes.
The clinical course of infection
Infection with HIV-1 is associated with a progressive loss of CD4 T cells (lymphocytes). This rate of loss can be measured and is used to determine the stage of infection. The loss of CD4 T cells is linked with an increase in viral load. HIV plasma levels during all stages of infection range from just 50 to 11 million virions per mL.
There are four stages of HIV infection: primary infection (or viremia or acute infection) which progresses over time to clinical latency (where the virus is a provirus inside monocytes) and then to symptomatic HIV infection, and finally, AIDS which is identified on the basis of certain infections, an HIV test and a CD4 T cell count.
Primary infection

Primary, or acute infection is a period of rapid viral replication that immediately follows the individual's exposure to HIV. During primary HIV infection, most individuals (80 to 90%) develop an acute syndrome characterised by flu-like symptoms of fever, malaise, lymphadenopathy, pharyngitis, headache, myalgia, and sometimes a rash. Within an average of three weeks after transmission of HIV-1, a broad HIV-1 specific immune response occurs that includes seroconversion. Because of the nonspecific nature of these illnesses, it is often not recognized as a sign of HIV infection. Even if patients go to their doctors or a hospital, they will often be misdiagnosed as having one of the more common infectious diseases with the same symptoms. Since not all patients develop it, and since the same symptoms can be caused by many other common diseases, it cannot be used as an indicator of HIV infection. However, recognizing the syndrome is important because the patient is much more infectious during this period. The CD4 T cell count is still higher than 1000 cells per µL.
Clinical latency
A strong immune defense reduces the number of viral particles in the blood stream, marking the start of the infection's clinical latency stage. Clinical latency can vary between two weeks and 20 years. During this early phase of infection, HIV is active within lymphoid organs, where large amounts of virus become trapped in the follicular dendritic cells (FDC) network. The surrounding tissues that are rich in CD4 T cells may also become infected, and viral particles accumulate both in infected cells and as free virus. Individuals who have entered into this phase are still infectious. The CD4 T cell count is normally at or around 1000 cells per µL. During this time, the most prevalent cell infected is the macrophage.
Symptoms of early infection
Further information: WHO Disease Staging System for HIV Infection and DiseaseThe first symptoms of HIV infection often include moderate and unexplained weight loss, recurrent respiratory tract infections (such as sinusitis, bronchitis, otitis media, pharyngitis), herpes zoster and recurrent oral ulcerations.
With the progression of the illness, other symptoms may start to present. These include unexplained chronic diarrhoea and persistent fever (for longer than one month), severe weight loss (>10% of presumed or measured body weight), oral hairy leukoplakia and candidiasis and severe bacterial infections including pulmonary tuberculosis. It is during this period that the CD4 T cells count starts to fall below 500 cells per µL.
The declaration of AIDS
- For more details on this topic, see AIDS Diagnosis and AIDS Symptoms and Complications.
AIDS is the most severe manifestation of infection with HIV and occurs when the CD4 T cell count drops to below 200 cells per µL. Classical symptoms are often severe opportunistic infections, rare cancers, neurological complications, and malnutrition. These include:
- Kaposi's sarcoma - a cancer of the blood vessels that accounts for extensive "bruising" sometimes seen in AIDS patients. It is aggressive, and can attack the mouth, lymph nodes, and internal organs, which may include the lungs or gastrointestinal tract.
- Pneumocystis jiroveci pneumonia - a form of pneumonia caused by the yeast-like fungal microorganism Pneumocystis jiroveci (sometimes spelled jirovecii and formerly classified as Pneumocystis carinii).
- AIDS dementia complex - encephalopathy due to HIV-infected brain cells
- Toxoplasmosis, Progressive multifocal leukoencephalopathy, common bacterial infections (Salmonella, Shigella, Listeria, Campylobacter, or Escherichia coli) and uncommon opportunistic infections such as cryptosporidiosis, microsporidiosis, Mycobacterium avium complex (MAC) and cytomegalovirus (CMV) colitis.
HIV test
Main article: HIV testApproximately half of those infected do not know their HIV status until it has progressed to an AIDS diagnosis. At this point many people tend to show opportunistic infection, who then see a doctor, get an HIV test and discover they are HIV positive. Donor blood and blood products used in medicine and medical research are screened for HIV using such a test. Typical HIV tests, including the HIV enzyme immunoassay and the Western blot assay, detect HIV antibodies in serum, plasma, oral fluid, dried blood spot or urine of patients. However, the time between initial infection and the development of detectable antibodies against HIV can vary. This is called the window period. This is why it can take 6-12 months to seroconvert and test positive. There are tests to detect other HIV antigens, HIV-RNA, and HIV-DNA in order to detect HIV infection prior to the development of detectable antibodies. HIV tests to detect antibodies, antigens or ribonucleic acid (RNA) in serum, plasma, oral fluid, dried blood spot or urine have been approved by FDA for donor screening, diagnosis, prognosis and patient monitoring.
Treatment
Further information: Antiretroviral drugHIV infection is a chronic infectious disease that can be treated, but not yet cured. There are effective means of preventing complications and delaying progression to AIDS. At the present time, not all persons infected with HIV have progressed to AIDS, but it is generally believed that the majority will.
A combination of several antiretroviral agents, termed Highly Active Anti-Retroviral Therapy HAART, has been highly effective in reducing the number of HIV particles in the blood stream (as measured by a blood test called the viral load). This can improve T-cell counts. This is not a cure for HIV, and people on HAART with suppressed levels of HIV can still transmit the virus to others through sex or sharing of needles. There is good evidence that if the levels of HIV remain suppressed and the CD4 count remains greater than 200, then the quality and length of life can be significantly improved and prolonged. Improved antiretroviral inhibitors against proteins such as Reverse transcriptase, Integrase and Tat are being researched and developed. One of the most promising new therapies is a new class of drugs called fusion or entry inhibitors.
Current drug classes include NNRTI, NRTI, NtRTI, PI, FI. Each of the drugs in these classes represent individual pharmacodynamics and toxicities, requiring expert knowledge to appropriately select and dose an effective combination. Current recommendations for treatment include at least a three drug effective regime to induce complete cease of viral replication and reduce the incidence of new mutations. On July 12, 2006 Atripla, a combination of the three widely used anti-retroviral drugs was fast-tracked by the FDA and will be made available for purchase in 15 other countries under a US international AIDS relief programme.
Post-exposure prophylaxis (PEP) with a course of antiviral drugs is also thought to reduce the risk of seroconversion after high risk exposure (unprotected anal or vaginal sex) to HIV. To be effective, it must be started as soon as possible after exposure and no later than 72 hours post-exposure. The treatment for HIV lasts four weeks. While there is compelling data to suggest that PEP after HIV exposure is extremely effective, there have been cases where it has failed.
As yet, no vaccine has been developed to prevent HIV infection or disease in people who are not yet infected with HIV, but the potential worldwide public health benefits of such a preventive vaccine are vast. Researchers in many countries are seeking to produce such a vaccine, including through the International AIDS Vaccine Initiative.
Epidemiology
Main article: AIDS pandemic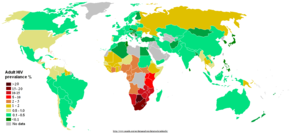
UNAIDS and the WHO estimate that AIDS has killed more than 25 million people since it was first recognized in 1981, making it one of the most destructive epidemics in recorded history. Despite recent, improved access to antiretroviral treatment and care in many regions of the world, the AIDS epidemic claimed an estimated 2.8 million (between 2.4 and 3.3 million) lives in 2005 of which more than half a million (570,000) were children.
Globally, between 33.4 and 46 million people currently live with HIV. In 2005, between 3.4 and 6.2 million people were newly infected and between 2.4 and 3.3 million people with AIDS died, an increase from 2004 and the highest number since 1981.
Sub-Saharan Africa remains by far the worst-affected region, with an estimated 21.6 to 27.4 million people currently living with HIV. Two million of them are children younger than 15 years of age. More than 64% of all people living with HIV are in sub-Saharan Africa, as are more than three quarters (76%) of all women living with HIV. In 2005, there were 12.0 million AIDS orphans living in sub-Saharan Africa 2005. South & South East Asia are second worst affected with 15%. AIDS accounts for the deaths of 500,000 children in this region. Two-thirds of HIV/AIDS infections in Asia occur in India, with an estimated 5.7 million infections (estimated 3.4—9.4 million) (0.9% of population), surpassing South Africa's estimated 5.5 million (4.9-6.1 million) (11.9% of population) infections, making it the country with the highest number of HIV infections in the world. In the 35 African nations with the highest prevalence, average life expectancy is 48.3 years—6.5 years less than it would be without the disease.
The latest evaluation report of the World Bank's Operations Evaluation Department assesses the development effectiveness of the World Bank's country-level HIV/AIDS assistance defined as policy dialogue, analytic work, and lending with the explicit objective of reducing the scope or impact of the AIDS epidemic. This is the first comprehensive evaluation of the World Bank's HIV/AIDS support to countries, from the beginning of the epidemic through mid-2004. Because the Bank's assistance is for implementation of government programmes by government, it provides important insights on how national AIDS programmes can be made more effective.
The development of HAART as effective therapy for HIV infection and AIDS has substantially reduced the death rate from this disease in those areas where it is widely available. This has created the misperception that the disease has gone away. In fact, as the life expectancy of persons with AIDS has increased in countries where HAART is widely used, the number of persons living with AIDS has increased substantially. In the United States, the number of persons with AIDS increased from about 35,000 in 1988 to over 220,000 in 1996.
In Africa, the number of MTCT and the prevalence of AIDS is beginning to reverse decades of steady progress in child survival. Countries such as Uganda are attempting to curb the MTCT epidemic by offering VCT (voluntary counselling and testing), PMTCT (prevention of mother-to-child transmission) and ANC (ante-natal care) services, which include the distribution of antiretroviral therapy.
Alternative theories
Main article: AIDS reappraisalSome scientists and activist groups question the connection between HIV and AIDS, the alleged danger of HIV, or the validity of current testing methods. These claims are met with resistance by, and often evoke frustration and hostility from, members of both the medical community, and the scientific community. These proponents for re-evaluation for HIV as the cause of AIDS bring forth such points as Koch's postulates, suggesting that since HIV is not present in 100% of AIDS cases, HIV can not be the sole cause of AIDS. HIV also does not always lead to AIDS when injected into a new, healthy host and be found to be growing again in this host. Dissidents assert that the current mainstream approach to AIDS, based on HIV causation, has resulted in inaccurate diagnoses, psychological terror, toxic treatments, and a squandering of public funds. The debate and controversy regarding this issue from the early 1980s to the present has provoked heated emotions and passions from both sides.
Notes and references
- ^ Joint United Nations Programme on HIV/AIDS (2006). "Overview of the global AIDS epidemic". 2006 Report on the global AIDS epidemic.
{{cite book}}:|access-date=requires|url=(help);|format=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - Joint United Nations Programme on HIV/AIDS. "AIDS epidemic update, 2005" (PDF format). Retrieved 2006-02-28.
{{cite web}}: Unknown parameter|publishyear=ignored (help) - Palella, F. J. Jr, Delaney, K. M., Moorman, A. C., Loveless, M. O., Fuhrer, J., Satten, G. A., Aschman and D. J., Holmberg, S. D. (1998). "Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators". N. Engl. J. Med. 338 (13): 853–860. PMID 9516219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - CDC (1981). "Pneumocystis Pneumonia --- Los Angeles". CDC. Retrieved 2006-01-17.
- Barré-Sinoussi, F., Chermann, J. C., Rey, F., Nugeyre, M. T., Chamaret, S., Gruest, J., Dauguet, C., Axler-Blin, C., Vezinet-Brun, F., Rouzioux, C., Rozenbaum, W. and Montagnier, L. (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 868–871. PMID 6189183.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Popovic, M., Sarngadharan, M. G., Read, E. and Gallo, R. C. (1984). "Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS". Science. 224 (4648): 497–500. PMID 6200935.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Coffin, J., Haase, A., Levy, J. A., Montagnier, L., Oroszlan, S., Teich, N., Temin, H., Toyoshima, K., Varmus, H., Vogt, P. and Weiss, R. A. (1986). "What to call the AIDS virus?". Nature. 321 (6065): 10. PMID 3010128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - International Committee on Taxonomy of Viruses. "61.0.6. Lentivirus". National Institutes of Health. Retrieved 2006-02-28.
{{cite web}}: Unknown parameter|publishyear=ignored (help) - International Committee on Taxonomy of Viruses. "61. Retroviridae". National Institutes of Health. Retrieved 2006-02-28.
{{cite web}}: Unknown parameter|publishyear=ignored (help) - Lévy, J. A. (1993). "HIV pathogenesis and long-term survival". AIDS. 7 (11): 1401–1410. PMID 8280406.
- Gao, F., Bailes, E., Robertson, D. L., Chen, Y., Rodenburg, C. M., Michael, S. F., Cummins, L. B., Arthur, L. O., Peeters, M., Shaw, G. M., Sharp, P. M., and Hahn, B. H. (1999). "Origin of HIV-1 in the Chimpanzee Pan troglodytes troglodytes". Nature. 397 (6718): 436–441. PMID 9989410 doi:10.1038/17130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Keele, B. F., van Heuverswyn, F., Li, Y. Y., Bailes, E., Takehisa, J., Santiago, M. L., Bibollet-Ruche, F., Chen, Y., Wain, L. V., Liegois, F., Loul, S., Mpoudi Ngole, E., Bienvenue, Y., Delaporte, E., Brookfield, J. F. Y., Sharp, P. M., Shaw, G. M., Peeters, M., and Hahn, B. H. (2006). "Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1". Science. Online 2006-05-25. doi:10.1126/science.1126531.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reeves, J. D. and Doms, R. W (2002). "Human Immunodeficiency Virus Type 2". J. Gen. Virol. 83 (Pt 6): 1253–1265. PMID 12029140.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Zhu, T., Korber, B. T., Nahmias, A. J., Hooper, E., Sharp, P. M. and Ho, D. D. (1998). "An African HIV-1 Sequence from 1959 and Implications for the Origin of the Epidemic". Nature. 391 (6667): 594–597. PMID 9468138 doi:10.1038/35400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Kolata, G. (1987-10-28). "Boy's 1969 death suggests AIDS invaded U.S. several times". The New York Times. Retrieved 2006-06-19.
{{cite news}}: Check date values in:|date=(help) - Hooper, E. (1997). "Sailors and star-bursts, and the arrival of HIV". BMJ. 315 (7123): 1689–1691. PMID 9448543.
- Curtis, T. (1992). "The origin of AIDS". Rolling Stone (626): 54–59, 61, 106, 108.
- Hooper, E. (1999). The River : A Journey to the Source of HIV and AIDS (1st ed.). Boston, MA: Little Brown & Co. pp. 1–1070. ISBN 0316372617.
- Smith, D. K., Grohskopf, L. A., Black, R. J., Auerbach, J. D., Veronese, F., Struble, K. A., Cheever, L., Johnson, M., Paxton, L. A., Onorato, I. A. and Greenberg, A. E. (2005). "Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV in the United States". MMWR. 54 (RR02): 1–20.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Donegan, E., Stuart, M., Niland, J. C., Sacks, H. S., Azen, S. P., Dietrich, S. L., Faucett, C., Fletcher, M. A., Kleinman, S. H., Operskalski, E. A.; et al. (1990). "Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations". Ann. Intern. Med. 113 (10): 733–739. PMID 2240875.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Coovadia, H. (2004). "Antiretroviral agents—how best to protect infants from HIV and save their mothers from AIDS". N. Engl. J. Med. 351 (3): 289–292. PMID 15247337.
- Kaplan, E. H. and Heimer, R. (1995). "HIV incidence among New Haven needle exchange participants: updated estimates from syringe tracking and testing data". J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10 (2): 175–176. PMID 7552482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ European Study Group on Heterosexual Transmission of HIV (1992). "Comparison of female to male and male to female transmission of HIV in 563 stable couples". BMJ. 304 (6830): 809–813. PMID 1392708.
- ^ Varghese, B., Maher, J. E., Peterman, T. A., Branson, B. M. and Steketee, R. W. (2002). "Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use". Sex. Transm. Dis. 29 (1): 38–43. PMID 11773877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bell, D. M. (1997). "Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview". Am. J. Med. 102 (5B): 9–15. PMID 9845490.
- Leynaert, B., Downs, A. M. and de Vincenzi, I. (1998). "Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV". Am. J. Epidemiol. 148 (1): 88–96. PMID 9663408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Siegfried, N., Muller, M., Deeks, J., Volmink, J., Egger, M., Low, N., Walker, S. and Williamson, P. (2005). "HIV and male circumcision--a systematic review with assessment of the quality of studies". Lancet Infect. Dis. 5 (3): 165–173. PMID 15766651.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - WHO (2005). "UNAIDS statement on South African trial findings regarding male circumcision and HIV". Retrieved 2006-03-28.
- Williams BG, Lloyd-Smith JO, Gouws E, Hankins C, Getz WM, Hargrove J, de Zoysa I, Dye C, Auvert B. (2006). "The Potential Impact of Male Circumcision on HIV in Sub-Saharan Africa". PLoS Med. 3 (7): e262. PMID 16822094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Various (2005). "Repeated Use of Unsterilized Blades in Ritual Circumcision Might Contribute to HIV Spread in S. Africa, Doctors Say". Kaisernetwork.org. Retrieved 2006-03-28.
- ^ Coakley, E., Petropoulos, C. J. and Whitcomb, J. M. (2005). "Assessing chemokine co-receptor usage in HIV". Curr. Opin. Infect. Dis. 18 (1): 9–15. PMID 15647694.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. (1996). "Identification of a major co-receptor for primary isolates of HIV-1". Nature. 381 (6584): 661–666. PMID 8649511.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Feng Y, Broder CC, Kennedy PE, Berger EA. (1996). "HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor". Science. 272 (5263): 872–877. PMID 8629022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Knight, S. C., Macatonia, S. E. and Patterson, S. (1990). "HIV I infection of dendritic cells". Int. Rev. Immunol. 6 (2–3): 163–175. PMID 2152500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tang, J. and Kaslow, R. A. (2003). "The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy". AIDS. 17 (Suppl 4): S51–S60. PMID 15080180.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. (1993). "Genotypic and phenotypic characterization of HIV-1 patients with primary infection". Science. 261 (5125): 1179–1181. PMID 8356453.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
van’t Wout AB, Kootstra NA, Mulder-Kampinga GA, Albrecht-van Lent N, Scherpbier HJ, Veenstra J, Boer K, Coutinho RA, Miedema F, Schuitemaker H. (1994). "Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission". J Clin Invest. 94 (5): 2060–2067. PMID 7962552.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD. (1996). "Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission". J Virol. 70 (5): 3098–3107. PMID 8627789.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Muciaccia B, Padula F, Vicini E, Gandini L, Lenzi A, Stefanini M. (2005). "Beta-chemokine receptors 5 and 3 are expressed on the head region of human spermatozoon". FASEB J. 19 (14): 2048–2050. PMID 16174786.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Berlier W, Bourlet T, Lawrence P, Hamzeh H, Lambert C, Genin C, Verrier B, Dieu-Nosjean MC, Pozzetto B, Delezay O. (2005). "Selective sequestration of X4 isolates by human genital epithelial cells: Implication for virus tropism selection process during sexual transmission of HIV". J Med Virol. 77 (4): 465–474. PMID 16254974.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Clevestig P, Maljkovic I, Casper C, Carlenor E, Lindgren S, Naver L, Bohlin AB, Fenyo EM, Leitner T, Ehrnst A. (2005). "The X4 phenotype of HIV type 1 evolves from R5 in two children of mothers, carrying X4, and is not linked to transmission". AIDS Res Hum Retroviruses. 5 (21): 371–378. PMID 15929699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Moore JP. (1997). "Coreceptors: implications for HIV pathogenesis and therapy". Science. 276 (5309): 51–52. PMID 9122710.
-
Karlsson A, Parsmyr K, Aperia K, Sandstrom E, Fenyo EM, Albert J. (1994). "MT-2 cell tropism of human immunodeficiency virus type 1 isolates as a marker for response to treatment and development of drug resistance". J Infect Dis. 170 (6): 1367–1375. PMID 7995974.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Koot M, van 't Wout AB, Kootstra NA, de Goede RE, Tersmette M, Schuitemaker H. (1996). "Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection". J Infect Dis. 173 (2): 349–354. PMID 8568295.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Chan, D. C. and Kim, P. S. (1998). "HIV entry and its inhibition". Cell. 93 (5): 681–684. PMID 9630213.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Wyatt, R. and Sodroski, J. (1998). "The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens". Science. 280 (5371): 1884–1888. PMID 9632381 doi:10.1126/science.280.5371.1884.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Zheng, Y. H., Lovsin, N. and Peterlin, B. M. (2005). "Newly identified host factors modulate HIV replication". Immunol. Lett. 97 (2): 225–234. PMID 15752562.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Pollard, V. W. and Malim, M. H. (1998). "The HIV-1 Rev protein". Annu. Rev. Microbiol. 52: 491–532. PMID 9891806.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gelderblom, H. R (1997). "Fine structure of HIV and SIV". In Los Alamos National Laboratory (ed.) (ed.). HIV Sequence Compendium. Los Alamos, New Mexico: Los Alamos National Laboratory. pp. 31–44.
{{cite book}}:|editor=has generic name (help);|format=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) -
Thomson, M. M., Perez-Alvarez, L. and Najera, R. (2002). "Molecular epidemiology of HIV-1 genetic forms and its significance for vaccine development and therapy". Lancet Infect. Dis. 2 (8): 461–471. PMID 12150845.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Carr, J. K. (1998). "Reference Sequences Representing the Principal Genetic Diversity of HIV-1 in the Pandemic". In Los Alamos National Laboratory (ed.) (ed.). HIV Sequence Compendium. Los Alamos, New Mexico: Los Alamos National Laboratory. pp. 10–19.
{{cite book}}:|editor=has generic name (help);|format=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) -
Osmanov S, Pattou C, Walker N, Schwardlander B, Esparza J; WHO-UNAIDS Network for HIV Isolation and Characterization. (2002). "Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000". Acquir. Immune. Defic. Syndr. 29 (2): 184–190. PMID 11832690.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Piatak, M., Jr, Saag, M. S., Yang, L. C., Clark, S. J., Kappes, J. C., Luk, K. C., Hahn, B. H., Shaw, G. M. and Lifson, J.D. (1993). "High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR". Science. 259 (5102): 1749–1754. PMID 8096089.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Kahn, J. O. and Walker, B. D. (1998). "Acute Human Immunodeficiency Virus type 1 infection". N. Engl. J. Med. 331 (1): 33–39. PMID 9647878.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bristol-Myers Squibb (2006). "U.S. Food And Drug Administration (FDA) Approves ATRIPLA™ (efavirenz 600 mg/ emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg), The First Once-Daily Single Tablet Regimen For Adults With HIV-1 Infection". Retrieved 2006-07-17.
- Fan, H., Conner, R. F. and Villarreal, L. P. eds, ed. (2005). AIDS : science and society (4th edition ed.). Boston, MA: Jones and Bartlett Publishers. ISBN 076370086X.
{{cite book}}:|edition=has extra text (help);|editor=has generic name (help); Cite has empty unknown parameter:|chapterurl=(help)CS1 maint: multiple names: editors list (link) - Joint United Nations Programme on HIV/AIDS (2006). "Annex 2: HIV/AIDS estimates and data, 2005". 2006 Report on the global AIDS epidemic.
{{cite book}}:|access-date=requires|url=(help);|format=requires|url=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - UNAIDS (2001). "Special Session of the General Assembly on HIV/AIDS Round table 3 Socio-economic impact of the epidemic and the strengthening of national capacities to combat HIV/AIDS" (PDF format). Retrieved 2006-06-15.
- World Bank (2005). "Evaluating the World Bank's Assistance for Fighting the HIV/AIDS Epidemic". Retrieved 2006-01-17.
- Centers for Disease Control and Prevention (1996). "U.S. HIV and AIDS cases reported through December 1996" (PDF format). HIV/AIDS Surveillance Report. 8 (2): 1–40.
- Duesberg, P. H. (1988). "HIV is not the cause of AIDS". Science. 241 (4865): 514, 517. PMID 3399880.
- Papadopulos-Eleopulos, E., Turner, V. F., Papadimitriou, J., Page, B., Causer, D., Alfonso, H., Mhlongo, S., Miller, T., Maniotis, A. and Fiala, C. (2004). "A critique of the Montagnier evidence for the HIV/AIDS hypothesis". Med Hypotheses. 63 (4): 597–601. PMID 15325002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Cohen, J. (1994). "The Duesberg phenomenon" (PDF format). Science. 266 (5191): 1642–1644. PMID 7992043.
External links
- POZ provides information and networking opportunities for the HIV community.
- AIDSmeds is a place to find complete and easy-to-read information on treating HIV & AIDS.
- HIV Medicine 2005, a medical textbook, >700 pages (free download)
- The Mechanism of HIV-1 Core Assembly: Insights from Three-Dimensional Reconstructions of Authentic Virions
- AIDS.ORG: Educating - Raising HIV Awareness - Building Community
- Be the Generation - Information on HIV Vaccine Clinical Research in 20 American Cities
- Capital Area Vaccine Effort
- AEGiS.org: AIDS Education Global Information System- Patient/clinician information & Historical news and treatment database
- AIDS/HIV Education
- Watch an animated tutorial on the life cycle of HIV
- Continuing medical education about HIV for healthcare providers
- FightAIDS@Home
- Everything you wanted to know about HIV and AIDS — Provided by New Scientist.
- Free HIV Images
- HIV/AIDS Treatment Information Service
- HIV/AIDS Education in Teacher Preparation Programs
- HIV InSite
- How Aids Works (with animation)
- Medecins Sans Frontieres/Doctors Without Borders HIV/AIDS Pages
- NIH/NIAD/DAIDS
- History of AIDS research at the NIH
- "The Molecules of HIV" information resource
- Unsafe Health Care and the HIV/AIDS Pandemic 2003
- HIV/AIDS: global trends, global funds and delivery bottlenecks
- UNAIDS document regarding three scenarios for HIV/AIDS in Africa for the year 2025 (Large PDF file)
- UNAIDS - Joint United Nations Programme on HIV/AIDS webpage
- Media Campaign: HIV leads to AIDS
- CSA54 - AIDS Cure News
- The role of dendritic cells in HIV pathogenesis
- HIV overview
- video ofTreatment Update 2006 from the Conference on Retrovirus'
- "The Age of Aids" March 30, 2006 Frontline
AIDS News
- Nov 2005 - Progress in HIV vaccine research -Recorded interview with Prof. Robert Gallo (HIV researcher)
- Jan 2006 - 3D structure of HIV is revealed - 3D map of AIDS causing virus revealed
- May 2006—HIV origin 'found in wild chimps'—HIV origin 'found in wild chimps'
