| Revision as of 10:29, 12 February 2016 editJDavidovits (talk | contribs)208 edits See comments in Talk. vandalistic action carried out by User Johnprovis since June 09, 2015 on the Geopolymer article. Consequently, we reverted the current issue to the last revision by Leyo dated of January 29, 2015← Previous edit | Revision as of 08:30, 13 February 2016 edit undoJohnprovis (talk | contribs)314 edits Undid revision 704588689 by JDavidovits (talk) You can't just revert a year's editing by all sorts of people (not just me) - needs to go through appropriate dispute resolution. See talk page.Next edit → | ||
| Line 1: | Line 1: | ||
| '''Geopolymers''' are |
'''Geopolymers''' are ], typically ceramic, materials that form long-range, covalently bonded, non-crystalline (]) networks . ] is an example of naturally-occurring geopolymer.{{citation needed|obsidian is a glass|date=January 2016}} Commercially produced geopolymers may be used for fire- and heat-resistant coatings and adhesives, medicinal applications, high-temperature ceramics, new binders for fire-resistant fiber composites, toxic and radioactive waste encapsulation and as cementing components to make concrete. The properties and uses of geopolymers are being explored in many scientific and industrial disciplines: modern inorganic chemistry, physical chemistry, ], mineralogy, geology, and in other types of engineering process technologies. Raw materials used in the synthesis of silicon-based polymers are mainly rock-forming minerals of geological origin, hence the name: ''geopolymer''. ] coined the term in 1978<ref name="IUPAC1982">An article published by the ] in 1982, outlines the reasons why the generic term ''geopolymer'' was chosen for this new chemistry. See: J. Davidovits, The Need to Create a New Technical Language For the Transfer of Basic Scientific Information, in ''Transfer and Exploitation of Scientific and Technical Information, Proceedings of the symposium, Luxemburg,'' 10–12 June 1981, pp. 316-320. It is available as a pdf-file and may be downloaded from the European Parliament Bookshop. Go to < http://bookshop.europa.eu/en/transfer-and-exploitation-of-scientific-and-technical-information-pbCD3381271/> and click on 'download'.</ref> and created the non profit French scientific institution (Association Loi 1901) ''Institut Géopolymère'' (Geopolymer Institute). | ||
| According to T.F. Yen<ref>Kim, D.; Lai, H.T.; Chilingar, G.V.; Yen T.F. (2006), Geopolymer formation and its unique properties, ''Environ. Geol,'' '''51''', 103–111.</ref> geopolymers can be classified into two major groups: pure inorganic geopolymers and organic containing geopolymers, synthetic analogues of naturally occurring ]s. In the following presentation, a geopolymer is essentially a mineral chemical compound or mixture of compounds consisting of repeating units, for example silico-oxide (-Si-O-Si-O-), silico-aluminate (-Si-O-Al-O-), ferro-silico-aluminate (-Fe-O-Si-O-Al-O-) or alumino-phosphate (-Al-O-P-O-), created through a process of geopolymerization.<ref>See |
According to T.F. Yen<ref>Kim, D.; Lai, H.T.; Chilingar, G.V.; Yen T.F. (2006), Geopolymer formation and its unique properties, ''Environ. Geol,'' '''51''', 103–111.</ref> geopolymers can be classified into two major groups: pure inorganic geopolymers and organic containing geopolymers, synthetic analogues of naturally occurring ]s. In the following presentation, a geopolymer is essentially a mineral chemical compound or mixture of compounds consisting of repeating units, for example silico-oxide (-Si-O-Si-O-), silico-aluminate (-Si-O-Al-O-), ferro-silico-aluminate (-Fe-O-Si-O-Al-O-) or alumino-phosphate (-Al-O-P-O-), created through a process of geopolymerization.<ref>See http://www.geopolymer.org/science/introduction</ref> This mineral synthesis (geosynthesis) was first presented at an ] symposium in 1976.<ref>Pdf-file #20 ''Milestone paper IUPAC 76'' at http://www.geopolymer.org/category/library/technical-papers</ref> | ||
| The microstructure of geopolymers is essentially temperature dependent: | The microstructure of geopolymers is essentially temperature dependent: | ||
| * It is X- |
* It is X-ray ] at room temperature, | ||
| * But |
* But evolves into a crystalline matrix at temperatures above 500 °C.<ref>Zoulgami, M; Lucas-Girot, A.; Michaud, V.; Briard, P.; Gaudé, J. and Oudadesse, H. (2002); Synthesis and physico-chemical characterization of a polysialate-hydroxyapatite composite for potential biomedical application, ''Eur. Phys. J. AP'', '''19''', 173-179. See also: Kriven, W.M.; Bell, J.; Gordon, M. (2003), Microstructure and Microchemistry of {{Sic|hide=y|Fully|-}}Reacted Geopolymers and Geopolymer Matrix Composites, ''Ceramic Transactions'', '''153''', 227–250; Perera, D.S. and Trautman R.L. (2005), Geopolymers with the Potential for Use as Refractory Castables, ''Advances in Technology of Materials and Materials Processing'', '''7''', 187–190.</ref> | ||
| One can distinguish between two synthesis routes: | One can distinguish between two synthesis routes: | ||
| * In ] medium (Na<sup>+</sup>, K<sup>+</sup>, Li<sup>+</sup>, Ca<sup> |
* In ] medium (Na<sup>+</sup>, K<sup>+</sup>, Li<sup>+</sup>, Ca<sup>2+</sup>, Cs<sup>+</sup> and the like); | ||
| * In acidic medium with ] and ]s. | * In acidic medium with ] and ]s. | ||
| Line 14: | Line 14: | ||
| The alkaline route is the most important in terms of R&D and commercial applications and will be described below. Details on the acidic route are to be found at the references<ref>Wagh, A.S. (2004), Chemically Bonded Phosphate Ceramics – A Novel Class of Geopolymers, ''Proceedings of the 106th Ann. Mtg. of the American Ceramic Society'', Indianapolis. See also, Chapter 13, Phosphate-based Geopolymers, in J. Davidovits' book ''Geopolymer Chemistry and Applications''.</ref> and<ref>Perera, D.S., Hanna, J.V., Davis, J., Blackford, M.G., Latella ,B.A., Sasaki ,Y. and Vance E.R. (2008), Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials, ''J. Mater. Sci.,'' '''43''', 6562–6566. See also, Cao, D.; Su, D.; Lu, B. and Yang Y. (2005), Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid, ''Journal Chinese Ceramic Society'', '''33''', 1385–89.</ref> | The alkaline route is the most important in terms of R&D and commercial applications and will be described below. Details on the acidic route are to be found at the references<ref>Wagh, A.S. (2004), Chemically Bonded Phosphate Ceramics – A Novel Class of Geopolymers, ''Proceedings of the 106th Ann. Mtg. of the American Ceramic Society'', Indianapolis. See also, Chapter 13, Phosphate-based Geopolymers, in J. Davidovits' book ''Geopolymer Chemistry and Applications''.</ref> and<ref>Perera, D.S., Hanna, J.V., Davis, J., Blackford, M.G., Latella ,B.A., Sasaki ,Y. and Vance E.R. (2008), Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials, ''J. Mater. Sci.,'' '''43''', 6562–6566. See also, Cao, D.; Su, D.; Lu, B. and Yang Y. (2005), Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid, ''Journal Chinese Ceramic Society'', '''33''', 1385–89.</ref> | ||
| == |
== What is a geopolymer? == | ||
| In the 1950s, Viktor Glukovsky, Kiev, |
In the 1950s, Viktor Glukovsky, of Kiev, USSR, developed concrete materials originally known under the names "soil silicate concretes" and "soil cements",<ref>Gluchovskij V.D.:"Gruntosilikaty" Gosstrojizdat Kiev 1959, ''Patent USSR'' 245 627 (1967), ''Patent USSR'' 449894 (Patent appl. 1958, granted 1974).</ref> but since the introduction of the geopolymer concept by ], the terminology and definitions of 'geopolymer' have become more diverse and often conflicting. The examples below were taken from 2011 scientific publications, written by scientists with different backgrounds. | ||
| '''''<big> |
'''''<big>Definitions of the term geopolymer</big>'''''<ref>See, Discussion at the Geopolymer Camp 2012, video ''Geopolymer definition in Misplaced Pages'' at http://www.geopolymer.org/camp/gp-camp-2012.</ref> | ||
| ''For chemists''<ref> |
''For chemists''<ref>Huang, Yi and Han, Minfang (2011) (China University of Mining and Technology, Beijing), The influence of α-Al<sub>2</sub>O<sub>3</sub> addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products, ''Journal of Hazardous Materials'', '''193''', 90–94</ref> | ||
| :'...It is known that alkali-activated aluminosilicates are able to produce alumino-silicate geopolymers. The hardening mechanism involves the chemical reaction of geopolymeric precursors, such as alumino-silicate oxides, with alkali polysilicates yielding polymeric Si–O–Al bonds.' | |||
| ''For geopolymer chemists''<ref>Huang, Yi and Han, Minfang (2011) (China University of Mining and Technology, Beijing), The influence of α-Al<sub>2</sub>O<sub>3</sub> addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products, ''Journal of Hazardous Materials'', '''193''', 90–94</ref> | |||
| :'...Geopolymers consist of a polymeric Si–O–Al framework, similar to zeolites. The main difference to zeolite is geopolymers are amorphous instead of crystalline. The microstructure of geopolymers on a nanometer scale observed by TEM comprises small aluminosilicate clusters with pores dispersed within a highly porous network. The clusters sizes are between 5 and 10 nanometers.' | :'...Geopolymers consist of a polymeric Si–O–Al framework, similar to zeolites. The main difference to zeolite is geopolymers are amorphous instead of crystalline. The microstructure of geopolymers on a nanometer scale observed by TEM comprises small aluminosilicate clusters with pores dispersed within a highly porous network. The clusters sizes are between 5 and 10 nanometers.' | ||
| ''For geopolymer material chemists''<ref>Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K. and Sato, T. (2011) (Department of Industrial Chemistry, Chiang Mai University, Thailand; CSIRO, Melbourne, Australia; Tohoku University, Sendai, Japan), Lightweight geopolymer made of highly porous siliceous materials with various Na<sub>2</sub>O/Al<sub>2</sub>O<sub>3</sub> and SiO<sub>2</sub>/Al<sub>2</sub>O<sub>3</sub> ratios, ''Materials Science and Engineering A'', '''528''', 6616–6623.</ref> | ''For geopolymer material chemists''<ref>Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K. and Sato, T. (2011) (Department of Industrial Chemistry, Chiang Mai University, Thailand; CSIRO, Melbourne, Australia; Tohoku University, Sendai, Japan), Lightweight geopolymer made of highly porous siliceous materials with various Na<sub>2</sub>O/Al<sub>2</sub>O<sub>3</sub> and SiO<sub>2</sub>/Al<sub>2</sub>O<sub>3</sub> ratios, ''Materials Science and Engineering A'', '''528''', 6616–6623.</ref> | ||
| :'...The reaction produces SiO<sub>4</sub> and AlO<sub>4</sub>, tetrahedral frameworks linked by shared oxygens as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo) depending on the SiO<sub>2</sub>/Al<sub>2</sub>O<sub>3</sub> ratio in the system. The connection of the tetrahedral frameworks is occurred via long-range covalent bonds. Thus, geopolymer structure is perceived as dense amorphous phase consisting of semi-crystalline 3-D alumino-silicate microstructure.' | :'...The reaction produces SiO<sub>4</sub> and AlO<sub>4</sub>, tetrahedral frameworks linked by shared oxygens as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo) depending on the SiO<sub>2</sub>/Al<sub>2</sub>O<sub>3</sub> ratio in the system. The connection of the tetrahedral frameworks is occurred via long-range covalent bonds. Thus, geopolymer structure is perceived as dense amorphous phase consisting of semi-crystalline 3-D alumino-silicate microstructure.' | ||
| ''For geopolymer ceramic chemists''<ref>Peigang He, Dechang Jia , Meirong Wang, Yu Zhou, (2011) (Harbin Institute of Technology, Harbin, PR China:), Thermal evolution and crystallization kinetics of potassium-based geopolymer, ''Ceramics International'', '''37''', 59–63.</ref> | |||
| :'...Although geopolymer is generally X-ray amorphous if cured at standard pressures and temperatures, it will convert into crystalline ceramic phases like leucite or pollucite upon heating.' | |||
| ''For alkali-cement scientists''<ref>Feng, Dingwu; Provis, John L. and van Deventer, Jannie S. J. (2012) (University of Melbourne, Australia), Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers, ''J. Am. Ceram. Soc.'', '''95''' 565–572.</ref> | ''For alkali-cement scientists''<ref>Feng, Dingwu; Provis, John L. and van Deventer, Jannie S. J. (2012) (University of Melbourne, Australia), Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers, ''J. Am. Ceram. Soc.'', '''95''' 565–572.</ref> | ||
| Line 39: | Line 33: | ||
| == Geopolymer synthesis == | == Geopolymer synthesis == | ||
| === |
=== Covalent bonding === | ||
| The fundamental unit within a geopolymer structure is a tetrahedral complex consisting of Si or Al coordinated through covalent bonds to four oxygens. The ''geopolymer'' framework results from the cross-linking between these tetrahedra, which leads to a 3-dimensional ] network, where the negative charge associated with tetrahedral aluminium is balanced by a small cationic species, most commonly an alkali metal cation. These alkali metal cations are often ], as they are associated with, but only loosely bonded to, the main covalent network, similarly to the non-framework cations present in ]s. | |||
| In 1937, W. L. Bragg published a method for classifying all kinds of ]s and their ]s based on the concept of the ionic theory by ]. The fundamental unit is a tetrahedral complex consisting of a small cation such as Si<sup>4+</sup>, or Al<sup>3+</sup> in tetrahedral coordination with four oxygens (Pauling’s first rule). Many textbooks explain the geometry of the SiO<sup>4−</sup> tetrahedron and other mineral structures as determined by the relative sizes of the different ions. | |||
| This ionic coordination representation is no longer adapted to the requirements of geopolymer chemistry that is governed by covalent bonding mechanisms. The differences between the ionic concept (coordination) and the covalent bonding are profound. The double tetrahedron structure (coordination) is sharing one oxygen anion O<sup>2−</sup>, whereas in the Si-O-Si- molecular structure, the covalent bond is achieved through Si and O co-sharing only one electron.<ref>See the figure at http://www.geopolymer.org/science/about-geopolymerization</ref> This results in stronger bond within the latter structure. The American mineralogist and geochemist G. V. Gibbs and his team studied the polymeric bond Si-O-Si-O and stated in 1982-2000: ''The successful modeling of the properties and structures of silica ... lends credence to the statement that a silica polymorph like quartz can be viewed as a giant molecule bound together by essentially the same forces that bind the atoms of the Si-O-Si skeleton into a small siloxane molecule''.<ref>Gibbs, G.V.; Hill, F.C.; Boisen Jr, M.B. and Downs R.T., (2000), ''Molecules as a Basis for Modeling the Force Field of Silica, Chapter 6 in Structure and Imperfections in Amorphous and Crystalline Silicon Dioxide'', Edited by R. A. B. Devine, J.-P. Duraud and E. Dooryhee, John Wiley & Sons Ltd</ref> The term ''giant molecule'' used by G.V. Gibbs is equivalent to the definition of ''geopolymer'' and the wording ''small siloxane molecule'' describes the actual ]s of organo-silicon compounds well known as ] polymer. These siloxane oligomers have the same structure as the silico-aluminate oligomers described below in this article. | |||
| === Geopolymerization starts with oligomers === | === Geopolymerization starts with oligomers === | ||
| ] | ] | ||
| Geopolymerization is the process of combining many small molecules known as oligomers into a covalently bonded network. The geo-chemical syntheses are carried out through oligomers (dimer, trimer, tetramer, pentamer) which |
Geopolymerization is the process of combining many small molecules known as ] into a covalently bonded network. The geo-chemical syntheses are carried out through oligomers (dimer, trimer, tetramer, pentamer) which are believed to contribute to the formation of the actual structure of the three-dimensional macromolecular framework, either through direct incorporation or through rearrangement via monomeric species. These oligomers are named by some geopolymer chemists as ''sialates'' following the scheme developed by Davidovits,<ref name="IUPAC1982" /> although this terminology is not universally accepted within the research community due in part to confusion with the earlier (1952) use of the same word to refer to the salts of the important biomolecule ].<ref>Provis, J.L. and Van Deventer, J.S.J. (2009), Introduction to geopolymers, in: ''Geopolymers: Structure, Processing, Properties and Industrial Applications'', J.L. Provis & Van Deventer (eds.), Woodhead, Cambridge UK, pp. 1-11</ref> In 2000, T.W. Swaddle and his team<ref>North, M.R. and Swaddle, T.W. (2000). Kinetics of Silicate Exchange in Alkaline Aluminosilicate Solutions, ''Inorg. Chem.'', '''39''', 2661–2665.</ref> proved the existence of soluble isolated alumino-silicate molecules in solution in relatively high concentrations and high ], at very low temperatures, as low as −9 °C. Indeed, it was discovered that the polymerization at room temperature of oligo-sialates was taking place on a time scale of around 100 milliseconds, i.e. 100 to 1000 times faster than the polymerization of ortho-silicate, oligo-siloxo units. At room temperature or higher, the reaction is so fast that it cannot be detected with conventional analytical equipment. | ||
| The image shows 5 soluble oligomers of the K-poly(sialate) / poly(sialate-siloxo) species, which are the actual starting units of potassium-based alumino-silicate geopolymerization. | The image shows 5 soluble oligomers of the K-poly(sialate) / poly(sialate-siloxo) species, which are the actual starting units of potassium-based alumino-silicate geopolymerization. | ||
| Line 54: | Line 47: | ||
| It involves four main phases comprising seven chemical reaction steps: | It involves four main phases comprising seven chemical reaction steps: | ||
| * Alkaline ] of the |
* Alkaline ] of the layered structure of the calcined ]; | ||
| * Formation of the ortho-sialate (OH)<sub>3</sub>-Si-O-Al-(OH)<sub>3</sub> molecule (#1 in the figure); | * Formation of monomeric and oligomeric species, including the "ortho-sialate" (OH)<sub>3</sub>-Si-O-Al-(OH)<sub>3</sub> molecule (#1 in the figure); | ||
| * In the presence of waterglass (soluble |
* In the presence of waterglass (soluble potassium silicate), cyclic Al-Si structures form (e.g. #5 in the figure), whereby the hydroxide is liberated by condensation reactions and can reacts again; | ||
| * Geopolymerization (]) into higher oligomers and polymeric 3D-networks. | * Geopolymerization (]) into higher oligomers and polymeric 3D-networks. | ||
| Line 67: | Line 60: | ||
| * These monomers inter-react to form ]s, which in turn react with other monomers to form trimers, tetramers and so on. | * These monomers inter-react to form ]s, which in turn react with other monomers to form trimers, tetramers and so on. | ||
| * When the solution reaches saturation, an aluminum-rich gel (denominated Gel 1) ]. | * When the solution reaches saturation, an aluminum-rich gel (denominated Gel 1) ]. | ||
| * As the reaction progresses, more Si-O groups |
* As the reaction progresses, more Si-O groups from the initial solid source dissolve, increasing the silicon concentration in the medium and gradually raising the proportion of silicon in the ] precursor gel (Gel 2). | ||
| * Polycondensation into zeolite-like 3D-frameworks. | * Polycondensation into zeolite-like 3D-frameworks. | ||
| Line 73: | Line 66: | ||
| ] | ] | ||
| Geopolymerization forms aluminosilicate frameworks that are similar to those of rock-forming minerals. Yet, there are major differences. In 1994, Davidovits<ref>Davidovits, J., (1994), Geopolymers: Man-Made Rock Geosynthesis and the Resulting Development of Very Early High Strength Cement, ''J. Materials Education'', '''16''' (2&3), 91–139.</ref> |
Geopolymerization forms aluminosilicate frameworks that are similar to those of rock-forming minerals. Yet, there are major differences. In 1994, Davidovits<ref>Davidovits, J., (1994), Geopolymers: Man-Made Rock Geosynthesis and the Resulting Development of Very Early High Strength Cement, ''J. Materials Education'', '''16''' (2&3), 91–139.</ref> presented a theoretical structure for K-poly(sialate-siloxo) (K)-(Si-O-Al-O-Si-O) that was consistent with the ]. It does not show the presence of water in the structure because he only focused on the relationship between Si, Al, Na, K, atoms. Water is present only at temperatures below 150 °C – 200 °C, whereas numerous geopolymer industrial and commercial applications work at temperatures above 200 °C, up to 1400 °C, i.e. at temperatures above ]. Nevertheless, scientists working on low temperature applications, such as cements and ], tried to pinpoint cation hydration and water molecules.<ref>Barbosa, V.F.F; MacKenzie, K.J.D. and Thaumaturgo, C., (2000), Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers, ''Intern. Journal of Inorganic Materials'', '''2''', pp. 309–317.</ref><ref>Rowles, M.R. (2004), The Structural Nature of Aluminosilicate Inorganic Polymers: a Macro to Nanoscale Study, ''PhD Thesis'', Curtin University of Technology, Perth, Australia.</ref> This model shows an incompletely reacted geopolymer (left in the figure), which involves free Si-OH groups that will later with time or with temperature polycondense with opposed Al-O-K, into Si-O-Al-O sialate bonds. The water released by this reaction either remains in the pores, is associated with the framework similarly to ] water, or can be released and removed. Several 3D-frameworks are described in the book 'Geopolymer Chemistry and Applications'.<ref>See: Structural frameworks and chemical mechanisms, in Davidovits' book Geopolymer Chemistry and Applications, Sections 8.6-8.7.</ref> After dehydroxylation (and dehydration), generally above 250 °C, geopolymers become more and more ] (right in the picture) and above 500-1000 °C (depending on the nature of the alkali cation present) crystallise and have X-ray diffraction patterns and framework structures identical to their geological analogues. | ||
| ==Commercial applications== | ==Commercial applications== | ||
| Line 98: | Line 91: | ||
| == Geopolymer resins and binders == | == Geopolymer resins and binders == | ||
| The class of geopolymer materials is described by Davidovits to comprise:<ref>see the Chapters 8, 11, 20 in J. Davidovits' book ''Geopolymer Chemistry and Applications''.</ref> | |||
| * '''Metakaolin MK-750-based geopolymer binder''' | * '''Metakaolin MK-750-based geopolymer binder''' | ||
| :chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 (range 1.5 to 2.5) | :chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 (range 1.5 to 2.5) | ||
| Line 106: | Line 99: | ||
| :chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 | :chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 | ||
| The first geopolymer resin was described in a French patent application filed by J. Davidovits in 1979. The American patent, US 4,349,386, was granted on Sept. 14, 1982 with the title ''Mineral Polymers and methods of making them''. It essentially involved the geopolymerization of alkaline soluble silicate with calcined kaolinitic clay (later coined ] MK-750 to |
The first geopolymer resin was described in a French patent application filed by J. Davidovits in 1979. The American patent, US 4,349,386, was granted on Sept. 14, 1982 with the title ''Mineral Polymers and methods of making them''. It essentially involved the geopolymerization of alkaline soluble silicate with calcined kaolinitic clay (later coined ] MK-750 to highlight the importance of the temperature of ], namely 750 °C in this case). In 1985, Kenneth MacKenzie and his team from New-Zealand, discovered the Al(V) coordination of calcined kaolinite (MK-750).<ref>MacKenzie, K.J.D.; Brown, I.W.M; Meinhold, R.H. and Bowden, M.E. (1985), Outstanding Problems in the Kaolinite-Mullite Reaction; Sequence Investigated by <sup>29</sup>Si and <sup>27</sup>Al Solid-State Nuclear Magnetic Resonance: I, Metakaolinite, ''J. Am. Ceram. Soc.'', '''68''' , 293–297.</ref> This had a great input towards a better understanding of its geopolymeric reactivity.{{disputed-inline|Mackenzie specifically said it wasn't 5-coordinated|date=June 2015}} | ||
| Since 1979, a variety of resins, binders and grouts were developed by the ], worldwide.<ref>see the updates in the Keynotes ''State of Geopolymer R&D'', 2009, 2010, 2011, and 2012 at http://www.geopolymer.org/camp)</ref> | Since 1979, a variety of resins, binders and grouts were developed by the ], worldwide.<ref>see the updates in the Keynotes ''State of Geopolymer R&D'', 2009, 2010, 2011, and 2012 at http://www.geopolymer.org/camp)</ref> | ||
| Line 112: | Line 105: | ||
| === Potential utilization for geopolymer composites materials === | === Potential utilization for geopolymer composites materials === | ||
| Metakaolin MK-750-based and Silica-based geopolymer resins are used to impregnate fibers and fabrics to obtain geopolymer matrix-based fiber composites. These products are |
Metakaolin MK-750-based and Silica-based geopolymer resins are used to impregnate fibers and fabrics to obtain geopolymer matrix-based fiber composites. These products are fire-resistant; they release no smoke and no toxic fumes. They were tested and recommended by major international institutions such as the American ] FAA.<ref>The FAA research project, 1994-1997 involved the collaboration between the research teams of: | ||
| – FAA Fire Department, Atlantic City, USA ; – Rutgers University of New Jersey, USA; – Cordi-Géopolymère laboratory, Saint-Quentin, France. A picture of geopolymer composite testing by FAA (Oil Burner Test of Fireproof composite) can be downloaded at http://www.fire.tc.faa.gov/research/targtare.stm | – FAA Fire Department, Atlantic City, USA ; – Rutgers University of New Jersey, USA; – Cordi-Géopolymère laboratory, Saint-Quentin, France. A picture of geopolymer composite testing by FAA (Oil Burner Test of Fireproof composite) can be downloaded at http://www.fire.tc.faa.gov/research/targtare.stm | ||
| </ref> FAA selected the carbon-geopolymer composite as the best candidate for the fire-resistant cabin program (1994-1997).<ref>Lyon, R.E.; Foden, A.J.; Balaguru, P.N.; Davidovits, J. and Davidovics, M. (1997), Properties of Geopolymer Matrix-Carbon Fiber Composites, ''Fire and Materials'', '''21''', 67–73.</ref> | </ref> FAA selected the carbon-geopolymer composite as the best candidate for the fire-resistant cabin program (1994-1997).<ref>Lyon, R.E.; Foden, A.J.; Balaguru, P.N.; Davidovits, J. and Davidovics, M. (1997), Properties of Geopolymer Matrix-Carbon Fiber Composites, ''Fire and Materials'', '''21''', 67–73.</ref> | ||
| === |
===Fire-resistant material=== | ||
| ] | ] | ||
| Line 125: | Line 118: | ||
| == Geopolymer cements == | == Geopolymer cements == | ||
| {{Advert|date=November 2013}} | |||
| {{main|Geopolymer cement}} | |||
| From a terminological point of view, '''geopolymer cement'''<ref>Davidovits, J., (1991), Geopolymers: Inorganic Polymeric New Materials, ''J. Thermal Analysis'', '''37''', 1633–1656. See also Chapter 24 in ''Geopolymer Chemistry and Applications'', Joseph Davidovits, Institut Géopolymère, Saint-Quentin, France, 2008, ISBN 9782951482050 (3rd ed., 2011).</ref> is a binding system that hardens at room temperature, like regular ]. If a geopolymer compound requires heat setting it may not be called geopolymer ] but rather geopolymer binder. | |||
| There is often confusion between the meanings of the two terms ''geopolymer cement'' and ''geopolymer concrete''. A cement is a binder whereas concrete is the composite material resulting from the addition of cement to stone aggregates. In other words, to produce concrete one purchases cement (generally ] or geopolymer cement) and adds it to the concrete batch. | |||
| ] | |||
| '''Geopolymer cement''' is being developed and utilised as an alternative to conventional Portland cement for use in transportation, infrastructure, construction and offshore applications. It relies on minimally processed natural materials or industrial byproducts to significantly reduce its ], while also being very resistant to many common concrete durability issues. | |||
| Production of geopolymer cement requires an aluminosilicate precursor material such as ] or ], a user-friendly alkaline reagent<ref>See the examples at the Geopolymer Institute page http://www.geopolymer.org/applications/geopolymer-cement</ref> (for example, sodium or potassium soluble silicates with a molar ratio MR SiO<sub>2</sub>:M<sub>2</sub>O ≥ 1.65, M being Na or K) and water (See the definition for "user-friendly" reagent below). Room temperature hardening is more readily achieved with the addition of a source of calcium cations, often ]. | |||
| Geopolymer cement is sometimes mixed up with alkali-activated cement and concrete. Despite more than 50 years of application in Eastern Europe after the development by G.V. Glukhovsky, alkali-activated materials are not sold to third parties as commercial cement. They are simply 'alkali-activated concretes'. On the contrary, geopolymer chemistry was from the start aimed at manufacturing binders and cements for various types of applications. For example the British company banah UK (http://www.banahuk.co.uk) sells its ''banah-Cem''™ as geopolymer cement, whereas the Australian company Zeobond (http://www.zeobond.com) markets its ''E-crete''™ as geopolymer concrete (not cement). | |||
| Geopolymer cements can be formulated to cure more rapidly than Portland-based cements; some mixes gain most of their ultimate strength within 24 hours. However, they must also set slowly enough that they can be mixed at a batch plant, either for precasting or delivery in a concrete mixer. Geopolymer cement also has the ability to form a strong chemical bond with ] rock-based ]. In March 2010, the US Department of Transportation Federal Highway Administration released a TechBrief titled ''Geopolymer Concrete'' that states:<ref>http://www.fhwa.dot.gov/pavement/pub_details.cfm?id=665</ref> ''The production of versatile, cost-effective geopolymer cements that can be mixed and hardened essentially like Portland cement represents a '''game changing''' advancement, revolutionizing the construction of transportation infrastructure and the building industry.'' | |||
| From a terminological point of view, cement is a binding system that hardens at room temperature, like regular portland cement. If a geopolymer compound requires heat setting it may not be called geopolymer cement but rather geopolymer binder. | |||
| '''''Geopolymer concrete''''' | |||
| === Portland cement chemistry vs geopolymer chemistry === | |||
| There is often confusion between the meanings of the terms 'geopolymer cement' and 'geopolymer concrete'. A cement is a binder, whereas concrete is the composite material resulting from the mixing and hardening of cement with water (or an alkaline solution in the case of geopolymer cement), and stone aggregates. Materials of both types (geopolymer cements and geopolymer concretes) are commercially available in various markets internationally <ref>http://www.banahuk.co.uk</ref> <ref>http://www.zeobond.com</ref> | |||
| === Portland cement chemistry vs Geopolymer cement chemistry === | |||
| ] | ] | ||
| ''Left:'' hardening of |
''Left:'' hardening of Portland cement (P.C.) through hydration of calcium silicate into calcium silicate hydrate (C-S-H) and portlandite, Ca(OH)<sub>2</sub>. | ||
| ''Right'' |
''Right:'' hardening (setting) of geopolymer cement (GP) through poly-condensation of potassium oligo-(sialate-siloxo) into potassium poly(sialate-siloxo) cross linked network. | ||
| ==== Alkali-activated materials vs. geopolymer cements. ==== | |||
| === CO<sub>2</sub> emission during manufacture === | |||
| The manufacture of portland cement ] involves the calcination of ] according to the reaction: | |||
| :5CaCO<sub>3</sub> + 2SiO<sub>2</sub> → (3CaO,SiO<sub>2</sub>)(2CaO,SiO<sub>2</sub>) + 5CO<sub>2</sub> | |||
| The production of 1 tonne of portland clinker directly generates 0.55 tonnes of chemical-CO<sub>2</sub> and requires the combustion of carbon-fuel to yield an additional 0.40 tonnes of carbon dioxide. | |||
| Geopolymerization chemistry requires appropriate terminologies and notions that are evidently different from those in use by Portland cement experts. On this matter, the ''Australian Geopolymer Alliance''<ref>http://www.geopolymers.com.au/science/geopolymerization</ref>{{dead link|date=January 2016}} outlines on its web site the following statement: "] ''developed the notion of a geopolymer (a Si/Al inorganic polymer) to better explain these chemical processes and the resultant material properties. To do so required a major shift in perspective, away from the classical crystalline hydration chemistry of conventional cement chemistry. To date this shift has not been well accepted by practitioners in the field of alkali activated cements who still tend to explain such reaction chemistry in Portland cement terminology''. | |||
| :'''To simplify:''' ''1 T of portland cement = 0.95 T of carbon dioxide'' | |||
| Indeed, geopolymer cement is sometimes mixed up with alkali-activated cement and concrete, developed more than 50 years ago by V.D. Glukhovsky in Ukraine, the former Soviet Union.<ref>Gluchovskij V.D.:"Gruntosilikaty" Gosstrojizdat Kiev 1959, Patent USSR 245 627 (1967), Patent USSR 449894 (Patent appl. 1958, granted 1974).</ref> They were originally known under the names "soil silicate concretes" and "soil cements". Because Portland cement concretes can be affected by the deleterious ''Alkali-aggregate reaction'', coined AAR or ] coined ASR (see for example the RILEM Committee 219-ACS Aggregate Reaction in Concrete Structures <ref>http://www.rilem.org/gene/main.php?base=8750&gp_id=229</ref>), the wording ''alkali-activation'' sometimes has a negative impact on civil engineers. However, geopolymer cements do not in general show these deleterious reactions (see below in Properties), when an appropriate aggregate is selected. Terminology related to ''alkali-activated materials'' or ''alkali-activated geopolymers'' is also in wide (but debated) use. These cements, sometimes abbreviated AAM, encompass the specific fields of alkali-activated slags, alkali-activated coal ]es, and various blended cementing systems (see RILEM Technical committee 247-DTA).<ref>http://www.rilem.org/gene/main.php?base=8750&gp_id=290</ref> | |||
| On the opposite, geopolymer cements do not rely on calcium carbonate and generate much less CO<sub>2</sub> during manufacture, i.e. a reduction in the range of 40% to 80-90%.<ref>Davidovits, J. (1993), Carbon-Dioxide Greenhouse-Warming: What Future for portland Cement, ''Emerging Technologies Symposium on Cements and Concretes in the Global Environment'', organized by the portland Cement Association, Chicago, Illinois, March 1993. See also the paper: Davidovits, J. (1993), Geopolymer Cements to minimize Carbon-Dioxide Greenhouse Warming, ''Ceramic Transactions'', '''37''', Cement-Based Materials: Present, Future and Environmental Aspects, pp. 165–182.</ref> For detailed calculation go to the article ]. | |||
| ==== User-friendly alkaline-reagents ==== | |||
| ] | |||
| Although geopolymerization does not rely on toxic organic solvents but only on water, it needs chemical ingredients that may be dangerous and therefore requires some safety procedures. Material Safety rules classify the alkaline products in two categories: corrosive products (named here: hostile) and irritant products (named here: friendly).{{citation needed|cite appropriate regulatory standards?|date=January 2016}} The two classes are recognizable through their respective logos. | |||
| The table lists some alkaline chemicals and their corresponding safety label.<ref>See in ref. 2</ref> The corrosive products must be handled with gloves, glasses and masks. They are ''user-hostile'' and cannot be implemented in mass applications without the appropriate safety procedures. In the second category one finds Portland cement or hydrated lime, typical mass products. Geopolymeric alkaline reagents belonging to this class may also be termed as ''User-friendly'', although the irritant nature of the alkaline component and the potential inhalation risk of powders still require the selection and use of appropriate ], as in any situation where chemicals or powders are handled. | |||
| The development of so-called ''alkali-activated-cements'' or ''alkali-activated geopolymers'' (the latter considered by some to be incorrect terminology), as well as several recipes found in the literature and on the Internet, especially those based on fly ashes, use alkali silicates with molar ratios SiO<sub>2</sub>:M<sub>2</sub>O below 1.20, or systems based on pure NaOH (8M or 12M). These conditions are not user-friendly for the ordinary labor force, and require careful consideration of personal protective equipment if employed in the field. Indeed, laws, regulations, and state directives push to enforce for more health protections and security protocols for workers’ safety. | |||
| Conversely, Geopolymer cement recipes employed in the field generally involve alkaline soluble silicates with starting molar ratios ranging from 1.45 to 1.95, particularly 1.60 to 1.85, i.e. ''user-friendly'' conditions. It may happen that for research, some laboratory recipes have molar ratios in the 1.20 to 1.45 range. | |||
| === Geopolymer cement categories === | === Geopolymer cement categories === | ||
| They comprise: | |||
| The categories comprise: | |||
| * Slag-based geopolymer cement.<ref>Davidovits, J. and Sawyer, J.L., (1985), Early high-strength mineral polymer, ''US Patent'' 4,509,985, 1985, filed February 22, 1984. The first commercial geopolymer cement was coined Pyrament 2000™ designed for repair and patching operations.</ref> | * Slag-based geopolymer cement.<ref>Davidovits, J. and Sawyer, J.L., (1985), Early high-strength mineral polymer, ''US Patent'' 4,509,985, 1985, filed February 22, 1984. The first commercial geopolymer cement was coined Pyrament 2000™ designed for repair and patching operations.</ref> | ||
| * Rock-based geopolymer cement.<ref>Gimeno, D.; Davidovits, J.; Marini, C.; Rocher, P.; Tocco, S.; Cara, S.; Diaz, N.; Segura, C. and Sistu, G. (2003), Development of silicate-based cement from glassy alkaline volcanic rocks: interpretation of preliminary data related to chemical- mineralogical composition of geologic raw materials. Paper in Spanish, ''Bol. Soc. Esp. Cerám. Vidrio'', '''42''', 69–78. .</ref> | * Rock-based geopolymer cement.<ref>Gimeno, D.; Davidovits, J.; Marini, C.; Rocher, P.; Tocco, S.; Cara, S.; Diaz, N.; Segura, C. and Sistu, G. (2003), Development of silicate-based cement from glassy alkaline volcanic rocks: interpretation of preliminary data related to chemical- mineralogical composition of geologic raw materials. Paper in Spanish, ''Bol. Soc. Esp. Cerám. Vidrio'', '''42''', 69–78. .</ref> | ||
| * Fly ash-based geopolymer cement | * Fly ash-based geopolymer cement | ||
| **type 1: alkali-activated fly ash geopolymer.<ref>Palomo, A.; Grutzeck, M.W. and Blanco, M.T. (1999), Alkali-activated fly ashes: a cement for the future, ''Cement Concrete Res'', '''29''', 1323–1329.</ref> | **type 1: alkali-activated fly ash geopolymer.<ref>Palomo, A.; Grutzeck, M.W. and Blanco, M.T. (1999), Alkali-activated fly ashes: a cement for the future, ''Cement Concrete Res'', '''29''', 1323–1329.</ref> | ||
| **type 2: slag/fly ash-based geopolymer cement.<ref>GEOASH (2004–2007), The GEOASH project was carried out with a financial grant from the Research Fund for Coal and Steel of the European Community |
**type 2: slag/fly ash-based geopolymer cement.<ref>GEOASH (2004–2007), The GEOASH project was carried out with a financial grant from the Research Fund for Coal and Steel of the European Community, contract number RFC-CR-04005. It involves: Antenucci D., ISSeP, Liège, Belgium; Nugteren H.and Butselaar- Orthlieb V., Delft University of Technology, Delft, The Netherlands; Davidovits J., Cordi-Géopolymère Sarl, Saint-Quentin, France; Fernández-Pereira C. and Luna Y., University of Seville, School of Industrial Engineering, Sevilla, Spain; Izquierdo and M., Querol X., CSIC, ], Barcelona, Spain.</ref><ref>Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H. and Fernández-Pereira, C., (2009), Coal fly ash-based geopolymers: microstructure and metal leaching, ''Journal of Hazardous Materials'', '''166''', 561–566.</ref><ref>See: Chapter 12 in J. Davidovits' book ''Geopolymer Chemistry and Applications''.</ref> | ||
| * Ferro-sialate-based geopolymer cement.<ref>Davidovits, J. et al., Geopolymer cement of the Calcium-Ferroaluminium silicate polymer type and production process, PCT patent publication WO 2012/056125.</ref> | * Ferro-sialate-based geopolymer cement.<ref>Davidovits, J. et al., Geopolymer cement of the Calcium-Ferroaluminium silicate polymer type and production process, PCT patent publication WO 2012/056125.</ref> | ||
| ====Slag-based geopolymer cement==== | ==== Slag-based geopolymer cement. ==== | ||
| :''Components'': metakaolin (MK-750) + blast furnace slag + alkali silicate (user-friendly). | |||
| : | |||
| :''Geopolymeric make-up:'' Si:Al = 2 in fact{{citation needed|date=January 2016}} solid solution of Si:Al=1, Ca-poly(di-sialate) (anorthite type) + Si:Al =3 , K-poly(sialate-disiloxo) (orthoclase type) and C-S-H Ca-silicate hydrate. | |||
| The first geopolymer cement developed in the 1980s was of the type (K,Na,Ca)-poly(sialate) (or slag-based geopolymer cement) and resulted from the research developments carried out by ] and J.L. Sawyer at |
The first geopolymer cement developed in the 1980s was of the type (K,Na,Ca)-poly(sialate) (or slag-based geopolymer cement) and resulted from the research developments carried out by ] and J.L. Sawyer at Lone Star Industries, USA and yielded the invention of Pyrament® cement. The American patent application was filed in 1984 and the patent US 4,509,985 was granted on April 9, 1985 with the title 'Early high-strength mineral polymer'. | ||
| ==== |
==== Rock-based geopolymer cement. ==== | ||
| The replacement of a certain amount of MK-750 with selected volcanic tuffs yields geopolymer cement with better properties and less CO<sub>2</sub> emission than the simple slag-based geopolymer cement.{{citation needed|date=January 2016}} | |||
| :''Manufacture components:'' metakaolin MK-750, blast furnace slag, volcanic tuffs (calcined or not calcined), mine tailings and alkali silicate (user-friendly). | |||
| : | |||
| :''Geopolymeric make-up:'' Si:Al = 3, in fact{{citation needed|date=January 2016}} solid solution of Si:Al=1 Ca-poly(di-sialate) (anorthite type) + Si:Al =3-5 (Na,K)-poly(silate-multisiloxo) and C-S-H Ca-silicate hydrate. Gravel | |||
| ==== Fly ash-based geopolymer cements ==== | |||
| Later on, in 1997, building on the works conducted on slag-based geopolymeric cements, on the one hand and on the synthesis of zeolites from fly ashes on the other hand, Silverstrim et al.<ref>Silverstrim, T.; Rostami, H.; Larralde, J.C and Samadi-Maybodi, A. (1997), Fly ash cementitious material and method of making a product, ''US Patent'' 5,601,643.</ref> and van Jaarsveld and van Deventer<ref>Van Jaarsveld, J.G.S., van Deventer, J.S.J. and Lorenzen L. (1997), The potential use of geopolymeric materials to immobilize toxic metals: Part I. Theory and Applications, ''Minerals Engineering'', '''10''' (7), 659–669.</ref> developed geopolymeric fly ash-based cements. Silverstrim et al. US Patent 5,601,643 was titled 'Fly ash cementitious material and method of making a product'. | Later on, in 1997, building on the works conducted on slag-based geopolymeric cements, on the one hand and on the synthesis of zeolites from fly ashes on the other hand, Silverstrim et al.<ref>Silverstrim, T.; Rostami, H.; Larralde, J.C and Samadi-Maybodi, A. (1997), Fly ash cementitious material and method of making a product, ''US Patent'' 5,601,643.</ref> and van Jaarsveld and van Deventer<ref>Van Jaarsveld, J.G.S., van Deventer, J.S.J. and Lorenzen L. (1997), The potential use of geopolymeric materials to immobilize toxic metals: Part I. Theory and Applications, ''Minerals Engineering'', '''10''' (7), 659–669.</ref> developed geopolymeric fly ash-based cements. Silverstrim et al. US Patent 5,601,643 was titled 'Fly ash cementitious material and method of making a product'. | ||
| '''Presently two types based on siliceous (EN 197) or Class F (ASTM C618) fly ashes:''' | |||
| * Type 1: alkali-activated fly ash geopolymer (user-hostile): | |||
| :In many cases requires heat curing at 60-80°C; not manufactured separately as a cement, but rather produced directly as a fly-ash based concrete. NaOH (user-hostile) + fly ash: partially-reacted fly ash particles embedded in an alumino-silicate gel with Si:Al= 1 to 2, zeolitic type (chabazite-Na and sodalite)structures. | |||
| * Type 2: slag/fly ash-based geopolymer cement (user-friendly): | |||
| :Room-temperature cement hardening. User-friendly silicate solution + blast furnace slag + fly ash: fly ash particles embedded in a geopolymeric matrix with Si:Al= 2, (Ca,K)-poly(sialate-siloxo). | |||
| ==== Ferro-sialate-based geopolymer cement ==== | |||
| The properties are similar to those of rock-based geopolymer cement but involve geological elements with high iron oxide content. The geopolymeric make up is of the type poly(ferro-sialate) (Ca,K)-(-Fe-O)-(Si-O-Al-O-). This user-friendly geopolymer cement is in the development and commercialization phase.<ref>See the Keynote Conference video of State of the Geopolymer R&D 2012 at http://www.geopolymer.org/camp/gp-camp-2012 , first section: Geopolymer Science as well as the third section Geopolymer Cements; present manufacturer of this cement is the company ''banah UK'' (http://www.banahuk.co.uk)</ref> | |||
| === CO<sub>2</sub> emissions during manufacture === | |||
| According to the Australian concrete expert B. V. Rangan, the growing worldwide demand for concrete is a great opportunity for the development of geopolymer cements of all types, with their much lower tally of carbon dioxide CO<sub>2</sub>.<ref>Rangan, B.V., (2008), Low-Calcium Fly Ash-Based Geopolymer Concrete, Chapter 26 in ''Concrete Construction Engineering Handbook'', Editor-in-Chief E.G. Nawy, Second Edition, CRC Press, New York.</ref> | |||
| ==== CO<sub>2</sub> emission during manufacture of Portland cement clinker ==== | |||
| The manufacture of Portland cement ] involves the calcination of ] according to the reactions: | |||
| ::3CaCO<sub>3</sub> + SiO<sub>2</sub> → Ca<sub>3</sub>SiO<sub>5</sub> + 3CO<sub>2</sub> | |||
| ::2CaCO<sub>3</sub> + SiO<sub>2</sub> → Ca<sub>2</sub>SiO<sub>4</sub> + 2CO<sub>2</sub> | |||
| Reactions involving alumina also lead the formation of the aluminate and ferrite components of the clinker. | |||
| The production of 1 tonne of Portland clinker directly generates approximately 0.55 tonnes of chemical CO<sub>2</sub>, directly as a product of these reactions, and requires the combustion of carbonaceous fuel to yield approximately an additional 0.40 tonnes of carbon dioxide, although this is being reduced through gains in process efficiency and the use of waste as fuels. However, in total, 1 tonne of Portland cement leads to the emission of 0.8-1.0 tonnes of carbon dioxide.<ref>see section 5 of http://www.wbcsdcement.org/pdf/CSI%20GNR%20Report%20final%2018%206%2009.pdf</ref> | |||
| Conversely, '''Geopolymer cements''' do not rely on calcium carbonate as a key ingredient, and generate much less CO<sub>2</sub> during manufacture, i.e. a reduction in the range of 40% to 80-90%. ] delivered the first paper on this subject in March 1993 at a symposium organized by the American Portland Cement Association, Chicago, Illinois.<ref>Davidovits, J. (1993), Carbon-Dioxide Greenhouse-Warming: What Future for Portland Cement, ''Emerging Technologies Symposium on Cements and Concretes in the Global Environment''. See also ref. 25</ref> | |||
| The Portland cement industry reacted strongly by lobbying the legal institutions{{POV-check inline|date=January 2016}} so that they delivered CO<sub>2</sub> emission numbers, which did not include the part related to calcium carbonate decomposition, focusing only on combustion emission. An article written in the scientific magazine ''New Scientist'' in 1997 stated that: ''...estimates for CO<sub>2</sub> emissions from cement production have concentrated only on the former source . The UN’s Intergovernmental Panel on Climate Change puts the industry’s total contribution to CO<sub>2</sub> emissions at 2.4 %; the Carbon Dioxide Information Analysis Center at the Oak Ridge National Laboratory in Tennessee quotes 2.6 %. Now ] of the Geopolymer Institute... has for the first time looked at both sources. He has calculated that world cement production of 1.4 billion tonnes a year produces 7 % of current CO<sub>2</sub> emissions''.<ref>Pearce Fred, The concrete jungle overheats, ''New Scientist'', issue 2091 (19 July 1997), page 14); http://www.newscientist.com/article/mg15520912.200-the-concrete-jungle-overheats.html</ref> Fifteen years later (2012), the situation has worsened with Portland cement CO<sub>2</sub> emissions approaching 3 billion tonnes a year.<ref>See the video of the Keynote State of Geopolymer 2012, Section 3: Geopolymer Cements at time: 32 min, at http://www.geopolymer.org/camp/gp-camp-2012</ref> | |||
| The fact that the dangers to the world’s ecological system from the manufacture of Portland cement is so little known by politicians and public makes the problem all the more urgent: when nothing is known, nothing is done. | |||
| ==== Geopolymer Cements Energy Needs and CO<sub>2</sub> emissions ==== | |||
| This section compares the energy needs and CO<sub>2</sub> emissions for regular Portland cement, Rock-based Geopolymer Cements and Fly ash-based geopolymer cements. The comparison proceeds between Portland cement and geopolymer cements with similar strength, i.e. average 40 MPa at 28 days. There have been several studies published on the subject<ref>McLellan, B. C; Williams, R. P; Lay, J.; Arie van Riessen, A. and Corder G. D., (2011), Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement, ''Journal of Cleaner Production'', '''19''', 1080-1090</ref> that may be summarized in the following way: | |||
| '''Rock-based Geopolymer cement manufacture involves:''' | |||
| :* 70% by weight geological compounds (calcined at 700°C) | |||
| :* blast furnace slag | |||
| :* alkali-silicate solution (industrial chemical, user-friendly). | |||
| The presence of blast furnace slag provides room-temperature hardening and increases the mechanical strength. | |||
| {| class="wikitable centre" | |||
| |+ Energy needs and CO<sub>2</sub> emissions for 1 tonne of Portland cement and Rock-based Geopolymer cement.{{citation needed|date=January 2016}} | |||
| ! '''Energy needs (MJ/tonne)''' !! Calcination !! Crushing !! Silicate Sol. !! Total!! Reduction | |||
| |- | |||
| | Portland Cement || 4270 || 430 || 0 || 4700 || 0 | |||
| |- | |||
| | GP-cement, slag by-product || 1200 || 390 || 375 || 1965 || 59% | |||
| |- | |||
| | GP-cement, slag manufacture || 1950 || 390 || 375 || 2715 || 43% | |||
| |- | |||
| | '''CO<sub>2</sub> emissions (tonne)''' || || || || || | |||
| |- | |||
| | Portland Cement || 1.000 || 0.020 || || 1.020 || 0 | |||
| |- | |||
| | GP-cement, slag by-product || 0.140 || 0,018 || 0.050 || 0.208 || 80% | |||
| |- | |||
| | GP-cement, slag manufacture || 0.240 || 0.018 || 0.050 || 0.308 || 70% | |||
| |} | |||
| ''Energy needs'' | |||
| According to the US Portland Cement Association (2006){{citation needed|date=January 2016}}, energy needs for Portland cement is in the range of 4700 MJ/tonne (average). The calculation for Rock- based geopolymer cement is performed with following parameters: | |||
| ::- the blast furnace slag is available as by-product from the steel industry (no additional energy needed); | |||
| ::- or must be manufactured (re-smelting from non granulated slag or from geological resources). | |||
| In the most favorable case — slag availability as by-product — there is a reduction of 59% of the energy needs in the manufacture of Rock-based geopolymer-cement in comparison with Portland cement. In the least favorable case —slag manufacture — the reduction reaches 43%.{{citation needed|date=January 2016}} | |||
| ''CO<sub>2</sub> emissions during manufacture'' | |||
| In the most favorable case — slag availability as by-product — there is a reduction of 80% of the CO<sub>2</sub> emission during manufacture of Rock-based geopolymer cement in comparison with Portland cement. In the least favorable case —slag manufacture — the reduction reaches 70%. | |||
| '''Fly ash-based cements Class F fly ashes''' | |||
| They do not require any further heat treatment. The calculation is therefore easier. One achieves emissions in the range of 0.09 to 0.25 tonnes of CO<sub>2</sub> / 1 tonne of fly ash-based cement, i.e. CO<sub>2</sub> emissions that are reduced in the range of 75 to 90%. | |||
| === Properties for Rock-based geopolymer cement (Ca,K)-poly(sialate-disiloxo) === | |||
| See<ref>See Chapters 16 and 17 in book ''Geopolymer Chemistry and Applications'', Joseph Davidovits</ref> | |||
| * shrinkage during setting: < 0.05%, not measurable. | |||
| * compressive strength (uniaxial): > 90 MPa at 28 days (for high early strength formulation, 20 MPa after 4 hours). | |||
| * flexural strength: 10–15 MPa at 28 days (for high early strength of 10 MPa after 24 hours). | |||
| * Young Modulus: > 2 GPa. | |||
| * freeze-thaw: mass loss < 0.1% (ASTM D4842), strength loss <5 % after 180 cycles. | |||
| ] | |||
| * wet-dry: mass loss < 0.1% (ASTM D4843). | |||
| * leaching in water, after 180 days: K<sub>2</sub>O < 0.015%. | |||
| * water absorption: < 3%, not related to permeability. | |||
| * hydraulic permeability: 10<sup>−10</sup> m/s. | |||
| * sulfuric acid, 10%: mass loss 0.1% per day. | |||
| * hydrochloric acid, 5%: mass loss 1% per day. | |||
| * KOH 50%: mass loss 0.02% per day. | |||
| * ammonia solution: no observed mass loss. | |||
| * sulfate solution: shrinkage 0.02% at 28 days. | |||
| * alkali-aggregate reaction: no expansion after 250 days (-0.01 %), as shown in the figure, comparison with Portland cement (ASTM C227). These results were published in 1993.<ref>Davidovits, J., (1993), Geopolymer Cement to Minimize Carbon-dioxide Greenhouse- warming, in Cement-Based Materials: Present, Future and Environmental Aspects, ''Ceramic Transactions'', '''37''', 165–182.</ref> Geopolymer binders and cements even with alkali contents as high as 10%, do not generate any dangerous alkali-aggregate reaction when used with an aggregate of normal reactivity. <ref>Li, K.-L.; Huang, G.-H.; Chen, J.; Wang, D. and Tang, X.-S., (2005), Early Mechanical Property and Durability of Geopolymer, ''Geopolymer 2005 Proceedings'', 117–120. used another standard, ASTM C 441-97, by which powdered quartz glass is the reactive fine element. Portland cement mortars exhibited expansion at 90 days in the range of 0.9– 1.0% whereas geopolymer cement remained practically unchanged, with a small shrinkage of -0.03 % at 90 days.</ref> | |||
| == The need for standards == | |||
| In June 2012, the institution ] organized a symposium on Geopolymer Binder Systems. The introduction to the symposium states:{{citation needed|date=January 2016}} ''When performance specifications for Portland cement were written, non-portland binders were uncommon...New binders such as geopolymers are being increasingly researched, marketed as specialty products, and explored for use in structural concrete. This symposium is intended to provide an opportunity for ASTM to consider whether the existing cement standards provide, on the one hand, an effective framework for further exploration of geopolymer binders and, on the other hand, reliable protection for users of these materials''. | |||
| The existing Portland cement standards are not adapted to geopolymer cements. They must be created by an ''ad hoc'' committee. Yet, to do so, requires also the presence of standard geopolymer cements. Presently, every expert is presenting his own recipe based on local raw materials (wastes, by-products or extracted). There is a need for selecting the right geopolymer cement category. The 2012 State of the Geopolymer R&D,<ref>See the video at http://www.geopolymer.org/camp/gp-camp-2012</ref> suggested to select two categories, namely: | |||
| :* Type 2 slag/fly ash-based geopolymer cement: fly ashes are available in the major emerging countries; | |||
| :and | |||
| :* Ferro-sialate-based geopolymer cement: this geological iron rich raw material is present in all countries throughout the globe. | |||
| :and | |||
| :* the appropriate user-friendly geopolymeric reagent. | |||
| == Geopolymer applications to arts and archaeology == | == Geopolymer applications to arts and archaeology == | ||
| Line 177: | Line 298: | ||
| === Roman cements === | === Roman cements === | ||
| From the digging of ancient Roman ruins, one knows that approximately 95% of the concretes and mortars constituting the Roman buildings consist of a very simple lime cement, which hardened slowly through the precipitating action of carbon dioxide CO<sub>2</sub>, from the atmosphere and formation of ] ( |
From the digging of ancient Roman ruins, one knows that approximately 95% of the concretes and mortars constituting the Roman buildings consist of a very simple lime cement, which hardened slowly through the precipitating action of carbon dioxide CO<sub>2</sub>, from the atmosphere and formation of ] (C-S-H). This is a very weak to medium good material that was used essentially in the making of foundations and in buildings for the populace. | ||
| But for the building of their "ouvrages d’art", especially works related to water storage (cisterns, aqueducts), the Roman architects did not hesitate to use more sophisticated and expensive ingredients. These outstanding Roman cements are based on the calcic activation of ceramic aggregates (in Latin ''testa'', analogue to our modern metakaolin MK-750) and alkali rich volcanic tuffs (cretoni, zeolitic pozzolan), respectively with lime. MAS-NMR Spectroscopy investigations were carried out on these high-tech Roman cements dating to the 2nd century AD. They show their geopolymeric make-up.<ref>As part of the European research project GEOCISTEM , Davidovits J. and Davidovits F. sampled archaeological mortars and concretes dating back to the 2nd century AD and later, in Rome and Ostia, Italy. They selected two series of artifacts: ''Opus Signinum'' in Rome, ''Opus Caementicum / Testacaeum'': mortars and concretes (''carbunculus''), in Ostia. Partly published in ''Geopolymer ’99 Proceedings'', 283-295 and in Davidovits' book, ''Geopolymer Chemistry and Applications'', Section 17.4. See also the NMR spectra at: http://www.geopolymer.org/applications/archaeological-analogues-roman-cements</ref> | But for the building of their "ouvrages d’art", especially works related to water storage (cisterns, aqueducts), the Roman architects did not hesitate to use more sophisticated and expensive ingredients. These outstanding Roman cements are based on the calcic activation of ceramic aggregates (in Latin ''testa'', analogue to our modern metakaolin MK-750) and alkali rich volcanic tuffs (cretoni, zeolitic pozzolan), respectively with lime. MAS-NMR Spectroscopy investigations were carried out on these high-tech Roman cements dating to the 2nd century AD. They show their geopolymeric make-up.<ref>As part of the European research project GEOCISTEM , Davidovits J. and Davidovits F. sampled archaeological mortars and concretes dating back to the 2nd century AD and later, in Rome and Ostia, Italy. They selected two series of artifacts: ''Opus Signinum'' in Rome, ''Opus Caementicum / Testacaeum'': mortars and concretes (''carbunculus''), in Ostia. Partly published in ''Geopolymer ’99 Proceedings'', 283-295 and in Davidovits' book, ''Geopolymer Chemistry and Applications'', Section 17.4. See also the NMR spectra at: http://www.geopolymer.org/applications/archaeological-analogues-roman-cements</ref> | ||
| == See also == | |||
| * ] | |||
| ==References== | ==References== | ||
| Line 191: | Line 315: | ||
| ==External links== | ==External links== | ||
| * Geopolymer Institute: http://www.geopolymer.org/ | * Geopolymer Institute: http://www.geopolymer.org/ | ||
| * Geopolymer Alliance: http://www.geopolymers.com.au/. | * Geopolymer Alliance: http://www.geopolymers.com.au/.{{dead link|date=January 2016}} | ||
| ] | ] | ||
| Line 202: | Line 326: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Revision as of 08:30, 13 February 2016
Geopolymers are inorganic, typically ceramic, materials that form long-range, covalently bonded, non-crystalline (amorphous) networks . Obsidian is an example of naturally-occurring geopolymer. Commercially produced geopolymers may be used for fire- and heat-resistant coatings and adhesives, medicinal applications, high-temperature ceramics, new binders for fire-resistant fiber composites, toxic and radioactive waste encapsulation and as cementing components to make concrete. The properties and uses of geopolymers are being explored in many scientific and industrial disciplines: modern inorganic chemistry, physical chemistry, colloid chemistry, mineralogy, geology, and in other types of engineering process technologies. Raw materials used in the synthesis of silicon-based polymers are mainly rock-forming minerals of geological origin, hence the name: geopolymer. Joseph Davidovits coined the term in 1978 and created the non profit French scientific institution (Association Loi 1901) Institut Géopolymère (Geopolymer Institute).
According to T.F. Yen geopolymers can be classified into two major groups: pure inorganic geopolymers and organic containing geopolymers, synthetic analogues of naturally occurring macromolecules. In the following presentation, a geopolymer is essentially a mineral chemical compound or mixture of compounds consisting of repeating units, for example silico-oxide (-Si-O-Si-O-), silico-aluminate (-Si-O-Al-O-), ferro-silico-aluminate (-Fe-O-Si-O-Al-O-) or alumino-phosphate (-Al-O-P-O-), created through a process of geopolymerization. This mineral synthesis (geosynthesis) was first presented at an IUPAC symposium in 1976.
The microstructure of geopolymers is essentially temperature dependent:
- It is X-ray amorphous at room temperature,
- But evolves into a crystalline matrix at temperatures above 500 °C.
One can distinguish between two synthesis routes:
- In alkaline medium (Na, K, Li, Ca, Cs and the like);
- In acidic medium with phosphoric acid and humic acids.
The alkaline route is the most important in terms of R&D and commercial applications and will be described below. Details on the acidic route are to be found at the references and
What is a geopolymer?
In the 1950s, Viktor Glukovsky, of Kiev, USSR, developed concrete materials originally known under the names "soil silicate concretes" and "soil cements", but since the introduction of the geopolymer concept by Joseph Davidovits, the terminology and definitions of 'geopolymer' have become more diverse and often conflicting. The examples below were taken from 2011 scientific publications, written by scientists with different backgrounds.
Definitions of the term geopolymer
For chemists
- '...Geopolymers consist of a polymeric Si–O–Al framework, similar to zeolites. The main difference to zeolite is geopolymers are amorphous instead of crystalline. The microstructure of geopolymers on a nanometer scale observed by TEM comprises small aluminosilicate clusters with pores dispersed within a highly porous network. The clusters sizes are between 5 and 10 nanometers.'
For geopolymer material chemists
- '...The reaction produces SiO4 and AlO4, tetrahedral frameworks linked by shared oxygens as poly(sialates) or poly(sialate–siloxo) or poly(sialate–disiloxo) depending on the SiO2/Al2O3 ratio in the system. The connection of the tetrahedral frameworks is occurred via long-range covalent bonds. Thus, geopolymer structure is perceived as dense amorphous phase consisting of semi-crystalline 3-D alumino-silicate microstructure.'
For alkali-cement scientists
- '... Geopolymers are framework structures produced by condensation of tetrahedral aluminosilicate units, with alkali metal ions balancing the charge associated with tetrahedral Al. Conventionally, geopolymers are synthesized from a two-part mix, consisting of an alkaline solution (often soluble silicate) and solid aluminosilicate materials. Geopolymerization occurs at ambient or slightly elevated temperature, where the leaching of solid aluminosilicate raw materials in alkaline solutions leads to the transfer of leached species from the solid surfaces into a growing gel phase, followed by nucleation and condensation of the gel phase to form a solid binder.'
For ceramic scientists
- '...Geopolymers are a class of totally inorganic, alumino-silicate based ceramics that are charge balanced by group I oxides. They are rigid gels, which are made under relatively ambient conditions of temperature and pressure into near-net dimension bodies, and which can subsequently be converted to crystalline or glass-ceramic materials.'
Geopolymer synthesis
Covalent bonding
The fundamental unit within a geopolymer structure is a tetrahedral complex consisting of Si or Al coordinated through covalent bonds to four oxygens. The geopolymer framework results from the cross-linking between these tetrahedra, which leads to a 3-dimensional aluminosilicate network, where the negative charge associated with tetrahedral aluminium is balanced by a small cationic species, most commonly an alkali metal cation. These alkali metal cations are often ion-exchangeable, as they are associated with, but only loosely bonded to, the main covalent network, similarly to the non-framework cations present in zeolites.
Geopolymerization starts with oligomers
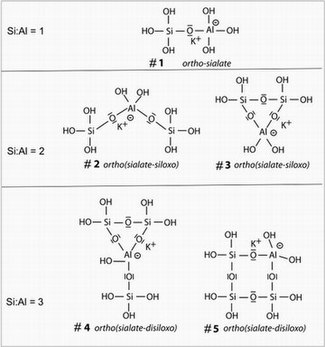
Geopolymerization is the process of combining many small molecules known as oligomers into a covalently bonded network. The geo-chemical syntheses are carried out through oligomers (dimer, trimer, tetramer, pentamer) which are believed to contribute to the formation of the actual structure of the three-dimensional macromolecular framework, either through direct incorporation or through rearrangement via monomeric species. These oligomers are named by some geopolymer chemists as sialates following the scheme developed by Davidovits, although this terminology is not universally accepted within the research community due in part to confusion with the earlier (1952) use of the same word to refer to the salts of the important biomolecule sialic acid. In 2000, T.W. Swaddle and his team proved the existence of soluble isolated alumino-silicate molecules in solution in relatively high concentrations and high pH, at very low temperatures, as low as −9 °C. Indeed, it was discovered that the polymerization at room temperature of oligo-sialates was taking place on a time scale of around 100 milliseconds, i.e. 100 to 1000 times faster than the polymerization of ortho-silicate, oligo-siloxo units. At room temperature or higher, the reaction is so fast that it cannot be detected with conventional analytical equipment.
The image shows 5 soluble oligomers of the K-poly(sialate) / poly(sialate-siloxo) species, which are the actual starting units of potassium-based alumino-silicate geopolymerization.
Example of (-Si-O-Al-O-) geopolymerization with metakaolin MK-750 in alkaline medium
It involves four main phases comprising seven chemical reaction steps:
- Alkaline depolymerization of the layered structure of the calcined kaolinite;
- Formation of monomeric and oligomeric species, including the "ortho-sialate" (OH)3-Si-O-Al-(OH)3 molecule (#1 in the figure);
- In the presence of waterglass (soluble potassium silicate), cyclic Al-Si structures form (e.g. #5 in the figure), whereby the hydroxide is liberated by condensation reactions and can reacts again;
- Geopolymerization (polycondensation) into higher oligomers and polymeric 3D-networks.
The geopolymerization kinetics for Na-poly(sialate-siloxo) and K-poly(sialate-siloxo) are slightly different respectively. This is probably due to the different dimensions of the Na and K cations, K being bigger than Na.
Example of zeolitic (Si-O-Al-O-) geopolymerization with fly ash in alkaline medium
It involves 5 main phases
- Nucleation stage in which the aluminosilicates from the fly ash particle dissolve in the alkaline medium (Na), releasing aluminates and silicates, probably as monomers.
- These monomers inter-react to form dimers, which in turn react with other monomers to form trimers, tetramers and so on.
- When the solution reaches saturation, an aluminum-rich gel (denominated Gel 1) precipitates.
- As the reaction progresses, more Si-O groups from the initial solid source dissolve, increasing the silicon concentration in the medium and gradually raising the proportion of silicon in the zeolite precursor gel (Gel 2).
- Polycondensation into zeolite-like 3D-frameworks.
Geopolymer 3D-frameworks
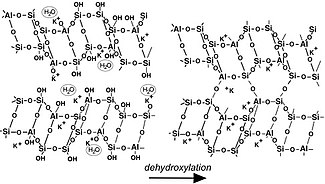
Geopolymerization forms aluminosilicate frameworks that are similar to those of rock-forming minerals. Yet, there are major differences. In 1994, Davidovits presented a theoretical structure for K-poly(sialate-siloxo) (K)-(Si-O-Al-O-Si-O) that was consistent with the NMR spectra. It does not show the presence of water in the structure because he only focused on the relationship between Si, Al, Na, K, atoms. Water is present only at temperatures below 150 °C – 200 °C, whereas numerous geopolymer industrial and commercial applications work at temperatures above 200 °C, up to 1400 °C, i.e. at temperatures above dehydroxylation. Nevertheless, scientists working on low temperature applications, such as cements and waste management, tried to pinpoint cation hydration and water molecules. This model shows an incompletely reacted geopolymer (left in the figure), which involves free Si-OH groups that will later with time or with temperature polycondense with opposed Al-O-K, into Si-O-Al-O sialate bonds. The water released by this reaction either remains in the pores, is associated with the framework similarly to zeolitic water, or can be released and removed. Several 3D-frameworks are described in the book 'Geopolymer Chemistry and Applications'. After dehydroxylation (and dehydration), generally above 250 °C, geopolymers become more and more crystalline (right in the picture) and above 500-1000 °C (depending on the nature of the alkali cation present) crystallise and have X-ray diffraction patterns and framework structures identical to their geological analogues.
Commercial applications
There exist a wide variety of potential and existing applications. Some of the geopolymer applications are still in development whereas others are already industrialized and commercialized. See the incomplete list provided by the Geopolymer Institute. They are listed in three major categories:
Geopolymer resins and binders
- Fire-resistant materials, thermal insulation, foams;
- Low-energy ceramic tiles, refractory items, thermal shock refractories;
- High-tech resin systems, paints, binders and grouts;
- Bio-technologies (materials for medicinal applications);
- Foundry industry (resins), tooling for the manufacture of organic fiber composites;
- Composites for infrastructures repair and strengthening, fire-resistant and heat-resistant high-tech carbon-fiber composites for aircraft interior and automobile;
- Radioactive and toxic waste containment;
Geopolymer cements and concretes
- Low-tech building materials (clay bricks),
- Low-CO2 cements and concretes;
Arts and archaeology
- Decorative stone artifacts, arts and decoration;
- Cultural heritage, archaeology and history of sciences.
Geopolymer resins and binders
The class of geopolymer materials is described by Davidovits to comprise:
- Metakaolin MK-750-based geopolymer binder
- chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2 (range 1.5 to 2.5)
- Silica-based geopolymer binder
- chemical formula (Na,K)-n(Si-O-)-(Si-O-Al-), ratio Si:Al>20 (range 15 to 40).
- Sol-gel-based geopolymer binder (synthetic MK-750)
- chemical formula (Na,K)-(Si-O-Al-O-Si-O-), ratio Si:Al=2
The first geopolymer resin was described in a French patent application filed by J. Davidovits in 1979. The American patent, US 4,349,386, was granted on Sept. 14, 1982 with the title Mineral Polymers and methods of making them. It essentially involved the geopolymerization of alkaline soluble silicate with calcined kaolinitic clay (later coined metakaolin MK-750 to highlight the importance of the temperature of calcination, namely 750 °C in this case). In 1985, Kenneth MacKenzie and his team from New-Zealand, discovered the Al(V) coordination of calcined kaolinite (MK-750). This had a great input towards a better understanding of its geopolymeric reactivity.
Since 1979, a variety of resins, binders and grouts were developed by the chemical industry, worldwide.
Potential utilization for geopolymer composites materials
Metakaolin MK-750-based and Silica-based geopolymer resins are used to impregnate fibers and fabrics to obtain geopolymer matrix-based fiber composites. These products are fire-resistant; they release no smoke and no toxic fumes. They were tested and recommended by major international institutions such as the American Federal Aviation Administration FAA. FAA selected the carbon-geopolymer composite as the best candidate for the fire-resistant cabin program (1994-1997).
Fire-resistant material

Flashover is a phenomenon unique to compartment fires where incomplete combustion products accumulate at the ceiling and ignite causing total involvement of the compartment materials and signaling the end to human survivability. Consequently, in a compartment fire the time to flashover is the time available for escape and this is the single most important factor in determining the fire hazard of a material or set of materials in a compartment fire. The Federal Aviation Administration has used the time-to-flashover of materials in aircraft cabin tests as the basis for a heat release and heat release rate acceptance criteria for cabin materials for commercial aircraft. The figure shows how the best organic-matrix made of engineering thermoplastics reaches flashover after the 20 minute ignition period and generates appreciable smoke, while the geopolymer-matrix composite will never ignite, reach flashover, or generate any smoke in a compartment fire.
Carbon-geopolymer composite is applied on racing cars around exhaust parts. This technology could be transferred and applied for the mass production of regular automobile parts (corrosion-resistant exhaust pipes and the like) as well as heat shields. A well-known motorcar manufacturer already developed a geopolymer-composite exhaust pipe system.
Geopolymer cements
| This article contains promotional content. Please help improve it by removing promotional language and inappropriate external links, and by adding encyclopedic text written from a neutral point of view. (November 2013) (Learn how and when to remove this message) |
From a terminological point of view, geopolymer cement is a binding system that hardens at room temperature, like regular Portland cement. If a geopolymer compound requires heat setting it may not be called geopolymer cement but rather geopolymer binder.

Geopolymer cement is being developed and utilised as an alternative to conventional Portland cement for use in transportation, infrastructure, construction and offshore applications. It relies on minimally processed natural materials or industrial byproducts to significantly reduce its carbon footprint, while also being very resistant to many common concrete durability issues.
Production of geopolymer cement requires an aluminosilicate precursor material such as metakaolin or fly ash, a user-friendly alkaline reagent (for example, sodium or potassium soluble silicates with a molar ratio MR SiO2:M2O ≥ 1.65, M being Na or K) and water (See the definition for "user-friendly" reagent below). Room temperature hardening is more readily achieved with the addition of a source of calcium cations, often blast furnace slag.
Geopolymer cements can be formulated to cure more rapidly than Portland-based cements; some mixes gain most of their ultimate strength within 24 hours. However, they must also set slowly enough that they can be mixed at a batch plant, either for precasting or delivery in a concrete mixer. Geopolymer cement also has the ability to form a strong chemical bond with silicate rock-based aggregates. In March 2010, the US Department of Transportation Federal Highway Administration released a TechBrief titled Geopolymer Concrete that states: The production of versatile, cost-effective geopolymer cements that can be mixed and hardened essentially like Portland cement represents a game changing advancement, revolutionizing the construction of transportation infrastructure and the building industry.
Geopolymer concrete
There is often confusion between the meanings of the terms 'geopolymer cement' and 'geopolymer concrete'. A cement is a binder, whereas concrete is the composite material resulting from the mixing and hardening of cement with water (or an alkaline solution in the case of geopolymer cement), and stone aggregates. Materials of both types (geopolymer cements and geopolymer concretes) are commercially available in various markets internationally
Portland cement chemistry vs Geopolymer cement chemistry

Left: hardening of Portland cement (P.C.) through hydration of calcium silicate into calcium silicate hydrate (C-S-H) and portlandite, Ca(OH)2.
Right: hardening (setting) of geopolymer cement (GP) through poly-condensation of potassium oligo-(sialate-siloxo) into potassium poly(sialate-siloxo) cross linked network.
Alkali-activated materials vs. geopolymer cements.
Geopolymerization chemistry requires appropriate terminologies and notions that are evidently different from those in use by Portland cement experts. On this matter, the Australian Geopolymer Alliance outlines on its web site the following statement: "Joseph Davidovits developed the notion of a geopolymer (a Si/Al inorganic polymer) to better explain these chemical processes and the resultant material properties. To do so required a major shift in perspective, away from the classical crystalline hydration chemistry of conventional cement chemistry. To date this shift has not been well accepted by practitioners in the field of alkali activated cements who still tend to explain such reaction chemistry in Portland cement terminology.
Indeed, geopolymer cement is sometimes mixed up with alkali-activated cement and concrete, developed more than 50 years ago by V.D. Glukhovsky in Ukraine, the former Soviet Union. They were originally known under the names "soil silicate concretes" and "soil cements". Because Portland cement concretes can be affected by the deleterious Alkali-aggregate reaction, coined AAR or Alkali-silica reaction coined ASR (see for example the RILEM Committee 219-ACS Aggregate Reaction in Concrete Structures ), the wording alkali-activation sometimes has a negative impact on civil engineers. However, geopolymer cements do not in general show these deleterious reactions (see below in Properties), when an appropriate aggregate is selected. Terminology related to alkali-activated materials or alkali-activated geopolymers is also in wide (but debated) use. These cements, sometimes abbreviated AAM, encompass the specific fields of alkali-activated slags, alkali-activated coal fly ashes, and various blended cementing systems (see RILEM Technical committee 247-DTA).
User-friendly alkaline-reagents

Although geopolymerization does not rely on toxic organic solvents but only on water, it needs chemical ingredients that may be dangerous and therefore requires some safety procedures. Material Safety rules classify the alkaline products in two categories: corrosive products (named here: hostile) and irritant products (named here: friendly). The two classes are recognizable through their respective logos.
The table lists some alkaline chemicals and their corresponding safety label. The corrosive products must be handled with gloves, glasses and masks. They are user-hostile and cannot be implemented in mass applications without the appropriate safety procedures. In the second category one finds Portland cement or hydrated lime, typical mass products. Geopolymeric alkaline reagents belonging to this class may also be termed as User-friendly, although the irritant nature of the alkaline component and the potential inhalation risk of powders still require the selection and use of appropriate personal protective equipment, as in any situation where chemicals or powders are handled.
The development of so-called alkali-activated-cements or alkali-activated geopolymers (the latter considered by some to be incorrect terminology), as well as several recipes found in the literature and on the Internet, especially those based on fly ashes, use alkali silicates with molar ratios SiO2:M2O below 1.20, or systems based on pure NaOH (8M or 12M). These conditions are not user-friendly for the ordinary labor force, and require careful consideration of personal protective equipment if employed in the field. Indeed, laws, regulations, and state directives push to enforce for more health protections and security protocols for workers’ safety.
Conversely, Geopolymer cement recipes employed in the field generally involve alkaline soluble silicates with starting molar ratios ranging from 1.45 to 1.95, particularly 1.60 to 1.85, i.e. user-friendly conditions. It may happen that for research, some laboratory recipes have molar ratios in the 1.20 to 1.45 range.
Geopolymer cement categories
The categories comprise:
- Slag-based geopolymer cement.
- Rock-based geopolymer cement.
- Fly ash-based geopolymer cement
- type 1: alkali-activated fly ash geopolymer.
- type 2: slag/fly ash-based geopolymer cement.
- Ferro-sialate-based geopolymer cement.
Slag-based geopolymer cement.
- Components: metakaolin (MK-750) + blast furnace slag + alkali silicate (user-friendly).
- Geopolymeric make-up: Si:Al = 2 in fact solid solution of Si:Al=1, Ca-poly(di-sialate) (anorthite type) + Si:Al =3 , K-poly(sialate-disiloxo) (orthoclase type) and C-S-H Ca-silicate hydrate.
The first geopolymer cement developed in the 1980s was of the type (K,Na,Ca)-poly(sialate) (or slag-based geopolymer cement) and resulted from the research developments carried out by Joseph Davidovits and J.L. Sawyer at Lone Star Industries, USA and yielded the invention of Pyrament® cement. The American patent application was filed in 1984 and the patent US 4,509,985 was granted on April 9, 1985 with the title 'Early high-strength mineral polymer'.
Rock-based geopolymer cement.
The replacement of a certain amount of MK-750 with selected volcanic tuffs yields geopolymer cement with better properties and less CO2 emission than the simple slag-based geopolymer cement.
- Manufacture components: metakaolin MK-750, blast furnace slag, volcanic tuffs (calcined or not calcined), mine tailings and alkali silicate (user-friendly).
- Geopolymeric make-up: Si:Al = 3, in fact solid solution of Si:Al=1 Ca-poly(di-sialate) (anorthite type) + Si:Al =3-5 (Na,K)-poly(silate-multisiloxo) and C-S-H Ca-silicate hydrate. Gravel
Fly ash-based geopolymer cements
Later on, in 1997, building on the works conducted on slag-based geopolymeric cements, on the one hand and on the synthesis of zeolites from fly ashes on the other hand, Silverstrim et al. and van Jaarsveld and van Deventer developed geopolymeric fly ash-based cements. Silverstrim et al. US Patent 5,601,643 was titled 'Fly ash cementitious material and method of making a product'.
Presently two types based on siliceous (EN 197) or Class F (ASTM C618) fly ashes:
- Type 1: alkali-activated fly ash geopolymer (user-hostile):
- In many cases requires heat curing at 60-80°C; not manufactured separately as a cement, but rather produced directly as a fly-ash based concrete. NaOH (user-hostile) + fly ash: partially-reacted fly ash particles embedded in an alumino-silicate gel with Si:Al= 1 to 2, zeolitic type (chabazite-Na and sodalite)structures.
- Type 2: slag/fly ash-based geopolymer cement (user-friendly):
- Room-temperature cement hardening. User-friendly silicate solution + blast furnace slag + fly ash: fly ash particles embedded in a geopolymeric matrix with Si:Al= 2, (Ca,K)-poly(sialate-siloxo).
Ferro-sialate-based geopolymer cement
The properties are similar to those of rock-based geopolymer cement but involve geological elements with high iron oxide content. The geopolymeric make up is of the type poly(ferro-sialate) (Ca,K)-(-Fe-O)-(Si-O-Al-O-). This user-friendly geopolymer cement is in the development and commercialization phase.
CO2 emissions during manufacture
According to the Australian concrete expert B. V. Rangan, the growing worldwide demand for concrete is a great opportunity for the development of geopolymer cements of all types, with their much lower tally of carbon dioxide CO2.
CO2 emission during manufacture of Portland cement clinker
The manufacture of Portland cement clinker involves the calcination of calcium carbonate according to the reactions:
- 3CaCO3 + SiO2 → Ca3SiO5 + 3CO2
- 2CaCO3 + SiO2 → Ca2SiO4 + 2CO2
Reactions involving alumina also lead the formation of the aluminate and ferrite components of the clinker.
The production of 1 tonne of Portland clinker directly generates approximately 0.55 tonnes of chemical CO2, directly as a product of these reactions, and requires the combustion of carbonaceous fuel to yield approximately an additional 0.40 tonnes of carbon dioxide, although this is being reduced through gains in process efficiency and the use of waste as fuels. However, in total, 1 tonne of Portland cement leads to the emission of 0.8-1.0 tonnes of carbon dioxide.
Conversely, Geopolymer cements do not rely on calcium carbonate as a key ingredient, and generate much less CO2 during manufacture, i.e. a reduction in the range of 40% to 80-90%. Joseph Davidovits delivered the first paper on this subject in March 1993 at a symposium organized by the American Portland Cement Association, Chicago, Illinois.
The Portland cement industry reacted strongly by lobbying the legal institutions so that they delivered CO2 emission numbers, which did not include the part related to calcium carbonate decomposition, focusing only on combustion emission. An article written in the scientific magazine New Scientist in 1997 stated that: ...estimates for CO2 emissions from cement production have concentrated only on the former source . The UN’s Intergovernmental Panel on Climate Change puts the industry’s total contribution to CO2 emissions at 2.4 %; the Carbon Dioxide Information Analysis Center at the Oak Ridge National Laboratory in Tennessee quotes 2.6 %. Now Joseph Davidovits of the Geopolymer Institute... has for the first time looked at both sources. He has calculated that world cement production of 1.4 billion tonnes a year produces 7 % of current CO2 emissions. Fifteen years later (2012), the situation has worsened with Portland cement CO2 emissions approaching 3 billion tonnes a year.
The fact that the dangers to the world’s ecological system from the manufacture of Portland cement is so little known by politicians and public makes the problem all the more urgent: when nothing is known, nothing is done.
Geopolymer Cements Energy Needs and CO2 emissions
This section compares the energy needs and CO2 emissions for regular Portland cement, Rock-based Geopolymer Cements and Fly ash-based geopolymer cements. The comparison proceeds between Portland cement and geopolymer cements with similar strength, i.e. average 40 MPa at 28 days. There have been several studies published on the subject that may be summarized in the following way:
Rock-based Geopolymer cement manufacture involves:
- 70% by weight geological compounds (calcined at 700°C)
- blast furnace slag
- alkali-silicate solution (industrial chemical, user-friendly).
The presence of blast furnace slag provides room-temperature hardening and increases the mechanical strength.
| Energy needs (MJ/tonne) | Calcination | Crushing | Silicate Sol. | Total | Reduction |
|---|---|---|---|---|---|
| Portland Cement | 4270 | 430 | 0 | 4700 | 0 |
| GP-cement, slag by-product | 1200 | 390 | 375 | 1965 | 59% |
| GP-cement, slag manufacture | 1950 | 390 | 375 | 2715 | 43% |
| CO2 emissions (tonne) | |||||
| Portland Cement | 1.000 | 0.020 | 1.020 | 0 | |
| GP-cement, slag by-product | 0.140 | 0,018 | 0.050 | 0.208 | 80% |
| GP-cement, slag manufacture | 0.240 | 0.018 | 0.050 | 0.308 | 70% |
Energy needs
According to the US Portland Cement Association (2006), energy needs for Portland cement is in the range of 4700 MJ/tonne (average). The calculation for Rock- based geopolymer cement is performed with following parameters:
- - the blast furnace slag is available as by-product from the steel industry (no additional energy needed);
- - or must be manufactured (re-smelting from non granulated slag or from geological resources).
In the most favorable case — slag availability as by-product — there is a reduction of 59% of the energy needs in the manufacture of Rock-based geopolymer-cement in comparison with Portland cement. In the least favorable case —slag manufacture — the reduction reaches 43%.
CO2 emissions during manufacture
In the most favorable case — slag availability as by-product — there is a reduction of 80% of the CO2 emission during manufacture of Rock-based geopolymer cement in comparison with Portland cement. In the least favorable case —slag manufacture — the reduction reaches 70%.
Fly ash-based cements Class F fly ashes
They do not require any further heat treatment. The calculation is therefore easier. One achieves emissions in the range of 0.09 to 0.25 tonnes of CO2 / 1 tonne of fly ash-based cement, i.e. CO2 emissions that are reduced in the range of 75 to 90%.
Properties for Rock-based geopolymer cement (Ca,K)-poly(sialate-disiloxo)
See
- shrinkage during setting: < 0.05%, not measurable.
- compressive strength (uniaxial): > 90 MPa at 28 days (for high early strength formulation, 20 MPa after 4 hours).
- flexural strength: 10–15 MPa at 28 days (for high early strength of 10 MPa after 24 hours).
- Young Modulus: > 2 GPa.
- freeze-thaw: mass loss < 0.1% (ASTM D4842), strength loss <5 % after 180 cycles.

- wet-dry: mass loss < 0.1% (ASTM D4843).
- leaching in water, after 180 days: K2O < 0.015%.
- water absorption: < 3%, not related to permeability.
- hydraulic permeability: 10 m/s.
- sulfuric acid, 10%: mass loss 0.1% per day.
- hydrochloric acid, 5%: mass loss 1% per day.
- KOH 50%: mass loss 0.02% per day.
- ammonia solution: no observed mass loss.
- sulfate solution: shrinkage 0.02% at 28 days.
- alkali-aggregate reaction: no expansion after 250 days (-0.01 %), as shown in the figure, comparison with Portland cement (ASTM C227). These results were published in 1993. Geopolymer binders and cements even with alkali contents as high as 10%, do not generate any dangerous alkali-aggregate reaction when used with an aggregate of normal reactivity.
The need for standards
In June 2012, the institution ASTM International organized a symposium on Geopolymer Binder Systems. The introduction to the symposium states: When performance specifications for Portland cement were written, non-portland binders were uncommon...New binders such as geopolymers are being increasingly researched, marketed as specialty products, and explored for use in structural concrete. This symposium is intended to provide an opportunity for ASTM to consider whether the existing cement standards provide, on the one hand, an effective framework for further exploration of geopolymer binders and, on the other hand, reliable protection for users of these materials.
The existing Portland cement standards are not adapted to geopolymer cements. They must be created by an ad hoc committee. Yet, to do so, requires also the presence of standard geopolymer cements. Presently, every expert is presenting his own recipe based on local raw materials (wastes, by-products or extracted). There is a need for selecting the right geopolymer cement category. The 2012 State of the Geopolymer R&D, suggested to select two categories, namely:
- Type 2 slag/fly ash-based geopolymer cement: fly ashes are available in the major emerging countries;
- and
- Ferro-sialate-based geopolymer cement: this geological iron rich raw material is present in all countries throughout the globe.
- and
- the appropriate user-friendly geopolymeric reagent.
Geopolymer applications to arts and archaeology
Because geopolymer artifacts look like natural stone, several artists started to cast in silicone rubber molds replications of their sculptures. For example, in the 1980s, the French artist Georges Grimal worked on several geopolymer castable stone formulations.
Egyptian Pyramid stones
Main article: Egyptian pyramid construction techniquesWith respects to archaeological applications, in the mid-1980s, Joseph Davidovits presented his first analytical results carried out on genuine pyramid stones. He claimed that the ancient Egyptians knew how to generate a geopolymeric reaction in the making of a re-agglomerated limestone blocks. The Ukrainian scientist G.V. Glukhovsky endorsed Davidovits' research in his keynote paper to the First Intern. Conf. on Alkaline Cements and Concretes, Kiev, Ukraine, 1994. Later on, several materials scientists and physicists took over these archaeological studies and are publishing their results, essentially on pyramid stones.
Roman cements
From the digging of ancient Roman ruins, one knows that approximately 95% of the concretes and mortars constituting the Roman buildings consist of a very simple lime cement, which hardened slowly through the precipitating action of carbon dioxide CO2, from the atmosphere and formation of calcium silicate hydrate (C-S-H). This is a very weak to medium good material that was used essentially in the making of foundations and in buildings for the populace.
But for the building of their "ouvrages d’art", especially works related to water storage (cisterns, aqueducts), the Roman architects did not hesitate to use more sophisticated and expensive ingredients. These outstanding Roman cements are based on the calcic activation of ceramic aggregates (in Latin testa, analogue to our modern metakaolin MK-750) and alkali rich volcanic tuffs (cretoni, zeolitic pozzolan), respectively with lime. MAS-NMR Spectroscopy investigations were carried out on these high-tech Roman cements dating to the 2nd century AD. They show their geopolymeric make-up.
See also
References
- ^ An article published by the Commission of the European Communities in 1982, outlines the reasons why the generic term geopolymer was chosen for this new chemistry. See: J. Davidovits, The Need to Create a New Technical Language For the Transfer of Basic Scientific Information, in Transfer and Exploitation of Scientific and Technical Information, Proceedings of the symposium, Luxemburg, 10–12 June 1981, pp. 316-320. It is available as a pdf-file and may be downloaded from the European Parliament Bookshop. Go to < http://bookshop.europa.eu/en/transfer-and-exploitation-of-scientific-and-technical-information-pbCD3381271/> and click on 'download'.
- Kim, D.; Lai, H.T.; Chilingar, G.V.; Yen T.F. (2006), Geopolymer formation and its unique properties, Environ. Geol, 51, 103–111.
- See http://www.geopolymer.org/science/introduction
- Pdf-file #20 Milestone paper IUPAC 76 at http://www.geopolymer.org/category/library/technical-papers
- Zoulgami, M; Lucas-Girot, A.; Michaud, V.; Briard, P.; Gaudé, J. and Oudadesse, H. (2002); Synthesis and physico-chemical characterization of a polysialate-hydroxyapatite composite for potential biomedical application, Eur. Phys. J. AP, 19, 173-179. See also: Kriven, W.M.; Bell, J.; Gordon, M. (2003), Microstructure and Microchemistry of Fully-Reacted Geopolymers and Geopolymer Matrix Composites, Ceramic Transactions, 153, 227–250; Perera, D.S. and Trautman R.L. (2005), Geopolymers with the Potential for Use as Refractory Castables, Advances in Technology of Materials and Materials Processing, 7, 187–190.
- Wagh, A.S. (2004), Chemically Bonded Phosphate Ceramics – A Novel Class of Geopolymers, Proceedings of the 106th Ann. Mtg. of the American Ceramic Society, Indianapolis. See also, Chapter 13, Phosphate-based Geopolymers, in J. Davidovits' book Geopolymer Chemistry and Applications.
- Perera, D.S., Hanna, J.V., Davis, J., Blackford, M.G., Latella ,B.A., Sasaki ,Y. and Vance E.R. (2008), Relative strengths of phosphoric acid-reacted and alkali-reacted metakaolin materials, J. Mater. Sci., 43, 6562–6566. See also, Cao, D.; Su, D.; Lu, B. and Yang Y. (2005), Synthesis and structure characterization of geopolymeric material based on metakaolinite and phosphoric acid, Journal Chinese Ceramic Society, 33, 1385–89.
- Gluchovskij V.D.:"Gruntosilikaty" Gosstrojizdat Kiev 1959, Patent USSR 245 627 (1967), Patent USSR 449894 (Patent appl. 1958, granted 1974).
- See, Discussion at the Geopolymer Camp 2012, video Geopolymer definition in Misplaced Pages at http://www.geopolymer.org/camp/gp-camp-2012.
- Huang, Yi and Han, Minfang (2011) (China University of Mining and Technology, Beijing), The influence of α-Al2O3 addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products, Journal of Hazardous Materials, 193, 90–94
- Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K. and Sato, T. (2011) (Department of Industrial Chemistry, Chiang Mai University, Thailand; CSIRO, Melbourne, Australia; Tohoku University, Sendai, Japan), Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios, Materials Science and Engineering A, 528, 6616–6623.
- Feng, Dingwu; Provis, John L. and van Deventer, Jannie S. J. (2012) (University of Melbourne, Australia), Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers, J. Am. Ceram. Soc., 95 565–572.
- Bell, Jonathan L.; Driemeyer, Patrick E. and Kriven, Waltraud M. (2009) (University of Illinois, USA), Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer, J. Am. Ceram. Soc., 92 , 607-615.
- Provis, J.L. and Van Deventer, J.S.J. (2009), Introduction to geopolymers, in: Geopolymers: Structure, Processing, Properties and Industrial Applications, J.L. Provis & Van Deventer (eds.), Woodhead, Cambridge UK, pp. 1-11
- North, M.R. and Swaddle, T.W. (2000). Kinetics of Silicate Exchange in Alkaline Aluminosilicate Solutions, Inorg. Chem., 39, 2661–2665.
- see at http://www.geopolymer.org/science/about-geopolymerization
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.l.; Lukey, G.C; Palomo, A. and Van Deventer, J.S.J., (2007), Geopolymer technology: the current state of the art, J. Mat. Sci., 42 (9) 2917–2933.
- Davidovits, J., (1994), Geopolymers: Man-Made Rock Geosynthesis and the Resulting Development of Very Early High Strength Cement, J. Materials Education, 16 (2&3), 91–139.
- Barbosa, V.F.F; MacKenzie, K.J.D. and Thaumaturgo, C., (2000), Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers, Intern. Journal of Inorganic Materials, 2, pp. 309–317.
- Rowles, M.R. (2004), The Structural Nature of Aluminosilicate Inorganic Polymers: a Macro to Nanoscale Study, PhD Thesis, Curtin University of Technology, Perth, Australia.
- See: Structural frameworks and chemical mechanisms, in Davidovits' book Geopolymer Chemistry and Applications, Sections 8.6-8.7.
- see at http://www.geopolymer.org/about/business-fellows
- see the Chapters 8, 11, 20 in J. Davidovits' book Geopolymer Chemistry and Applications.
- MacKenzie, K.J.D.; Brown, I.W.M; Meinhold, R.H. and Bowden, M.E. (1985), Outstanding Problems in the Kaolinite-Mullite Reaction; Sequence Investigated by Si and Al Solid-State Nuclear Magnetic Resonance: I, Metakaolinite, J. Am. Ceram. Soc., 68 , 293–297.
- see the updates in the Keynotes State of Geopolymer R&D, 2009, 2010, 2011, and 2012 at http://www.geopolymer.org/camp)
- The FAA research project, 1994-1997 involved the collaboration between the research teams of: – FAA Fire Department, Atlantic City, USA ; – Rutgers University of New Jersey, USA; – Cordi-Géopolymère laboratory, Saint-Quentin, France. A picture of geopolymer composite testing by FAA (Oil Burner Test of Fireproof composite) can be downloaded at http://www.fire.tc.faa.gov/research/targtare.stm
- Lyon, R.E.; Foden, A.J.; Balaguru, P.N.; Davidovits, J. and Davidovics, M. (1997), Properties of Geopolymer Matrix-Carbon Fiber Composites, Fire and Materials, 21, 67–73.
- Davidovics, M.; Bruno, M. and Davidovits, J. (1999), Past and Present Experience on the Use of Carbon-Géopolymère Composite in Formula One and CART Racing Cars, Geopolymer ’99 Proceedings, 141–142.
- Davidovits, J. (2002), 30 Years of Successes and Failures in Geopolymer Applications, Market Trends and Potential Breakthroughs, Geopolymer 2002 Conference, Oct. 28-29, Melbourne, Australia. Download the pdf-file #15 at http://www.geopolymer.org/category/library/technical-papers.
- See the PCT patent application publication WO 2004/106705 filed by Porsche AG, 2004.
- Davidovits, J., (1991), Geopolymers: Inorganic Polymeric New Materials, J. Thermal Analysis, 37, 1633–1656. See also Chapter 24 in Geopolymer Chemistry and Applications, Joseph Davidovits, Institut Géopolymère, Saint-Quentin, France, 2008, ISBN 9782951482050 (3rd ed., 2011).
- See the examples at the Geopolymer Institute page http://www.geopolymer.org/applications/geopolymer-cement
- http://www.fhwa.dot.gov/pavement/pub_details.cfm?id=665
- http://www.banahuk.co.uk
- http://www.zeobond.com
- http://www.geopolymers.com.au/science/geopolymerization
- Gluchovskij V.D.:"Gruntosilikaty" Gosstrojizdat Kiev 1959, Patent USSR 245 627 (1967), Patent USSR 449894 (Patent appl. 1958, granted 1974).
- http://www.rilem.org/gene/main.php?base=8750&gp_id=229
- http://www.rilem.org/gene/main.php?base=8750&gp_id=290
- See in ref. 2
- Davidovits, J. and Sawyer, J.L., (1985), Early high-strength mineral polymer, US Patent 4,509,985, 1985, filed February 22, 1984. The first commercial geopolymer cement was coined Pyrament 2000™ designed for repair and patching operations.
- Gimeno, D.; Davidovits, J.; Marini, C.; Rocher, P.; Tocco, S.; Cara, S.; Diaz, N.; Segura, C. and Sistu, G. (2003), Development of silicate-based cement from glassy alkaline volcanic rocks: interpretation of preliminary data related to chemical- mineralogical composition of geologic raw materials. Paper in Spanish, Bol. Soc. Esp. Cerám. Vidrio, 42, 69–78. .
- Palomo, A.; Grutzeck, M.W. and Blanco, M.T. (1999), Alkali-activated fly ashes: a cement for the future, Cement Concrete Res, 29, 1323–1329.
- GEOASH (2004–2007), The GEOASH project was carried out with a financial grant from the Research Fund for Coal and Steel of the European Community, contract number RFC-CR-04005. It involves: Antenucci D., ISSeP, Liège, Belgium; Nugteren H.and Butselaar- Orthlieb V., Delft University of Technology, Delft, The Netherlands; Davidovits J., Cordi-Géopolymère Sarl, Saint-Quentin, France; Fernández-Pereira C. and Luna Y., University of Seville, School of Industrial Engineering, Sevilla, Spain; Izquierdo and M., Querol X., CSIC, Institute of Earth Sciences Jaume Almera, Barcelona, Spain.
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H. and Fernández-Pereira, C., (2009), Coal fly ash-based geopolymers: microstructure and metal leaching, Journal of Hazardous Materials, 166, 561–566.
- See: Chapter 12 in J. Davidovits' book Geopolymer Chemistry and Applications.
- Davidovits, J. et al., Geopolymer cement of the Calcium-Ferroaluminium silicate polymer type and production process, PCT patent publication WO 2012/056125.
- Silverstrim, T.; Rostami, H.; Larralde, J.C and Samadi-Maybodi, A. (1997), Fly ash cementitious material and method of making a product, US Patent 5,601,643.
- Van Jaarsveld, J.G.S., van Deventer, J.S.J. and Lorenzen L. (1997), The potential use of geopolymeric materials to immobilize toxic metals: Part I. Theory and Applications, Minerals Engineering, 10 (7), 659–669.
- See the Keynote Conference video of State of the Geopolymer R&D 2012 at http://www.geopolymer.org/camp/gp-camp-2012 , first section: Geopolymer Science as well as the third section Geopolymer Cements; present manufacturer of this cement is the company banah UK (http://www.banahuk.co.uk)
- Rangan, B.V., (2008), Low-Calcium Fly Ash-Based Geopolymer Concrete, Chapter 26 in Concrete Construction Engineering Handbook, Editor-in-Chief E.G. Nawy, Second Edition, CRC Press, New York.
- see section 5 of http://www.wbcsdcement.org/pdf/CSI%20GNR%20Report%20final%2018%206%2009.pdf
- Davidovits, J. (1993), Carbon-Dioxide Greenhouse-Warming: What Future for Portland Cement, Emerging Technologies Symposium on Cements and Concretes in the Global Environment. See also ref. 25
- Pearce Fred, The concrete jungle overheats, New Scientist, issue 2091 (19 July 1997), page 14); http://www.newscientist.com/article/mg15520912.200-the-concrete-jungle-overheats.html
- See the video of the Keynote State of Geopolymer 2012, Section 3: Geopolymer Cements at time: 32 min, at http://www.geopolymer.org/camp/gp-camp-2012
- McLellan, B. C; Williams, R. P; Lay, J.; Arie van Riessen, A. and Corder G. D., (2011), Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement, Journal of Cleaner Production, 19, 1080-1090
- See Chapters 16 and 17 in book Geopolymer Chemistry and Applications, Joseph Davidovits
- Davidovits, J., (1993), Geopolymer Cement to Minimize Carbon-dioxide Greenhouse- warming, in Cement-Based Materials: Present, Future and Environmental Aspects, Ceramic Transactions, 37, 165–182.
- Li, K.-L.; Huang, G.-H.; Chen, J.; Wang, D. and Tang, X.-S., (2005), Early Mechanical Property and Durability of Geopolymer, Geopolymer 2005 Proceedings, 117–120. used another standard, ASTM C 441-97, by which powdered quartz glass is the reactive fine element. Portland cement mortars exhibited expansion at 90 days in the range of 0.9– 1.0% whereas geopolymer cement remained practically unchanged, with a small shrinkage of -0.03 % at 90 days.
- See the video at http://www.geopolymer.org/camp/gp-camp-2012
- See Potential utilizations in art and decoration, at http://www.geopolymer.org/applications/potential-utilizations-in-art-and-decoration ; a pdf article #19 Dramatized sculptures with geopolymers at http://www.geopolymer.org/category/library/technical-papers/
- Davidovits, J. (1986), X-Rays Analysis and X-Rays Diffraction of Casing Stones from the Pyramids of Egypt, and the Limestone of the Associated Quarries; pp. 511–20 in Science in Egyptology Symposia, Edited by R. A. David, Manchester University Press, Manchester, U.K. (Pdf-file #A in the Geopolymer Institute Library, Archaeological Papers); see also: Davidovits J., (1987), Ancient and modern concretes: what is the real difference? Concrete International: Des. Constr, 9 , 23–29. See also: Davidovits, J. and Morris, M., (1988), The Pyramids: An Enigma Solved. Hippocrene Books, New York, 1988.
- G.V Glukhovsky passed away before the conference. His keynote paper titled: Ancient, Modern and Future Concretes, is included in the Proceedings of the First Intern. Conf. on Alkaline Cements and Concretes, pp. 1-9, Kiev, Ukraine, 1994.
- Demortier ,G. (2004), PIXE, PIGE and NMR study of the masonry of the pyramid of Cheops at Giza, Nuclear Instruments and Methods, Physics Research B, 226, 98–109.
- Barsoum, M.W.; Ganguly, A. and Hug, G. (2006), Microstructural Evidence of Reconstituted Limestone Blocks in the Great Pyramids of Egypt, J. Am. Ceram. Soc. 89, 3788–3796.
- MacKenzie, Kenneth J.D.; Smith, Mark E.; Wong, Alan; Hanna, John V.; Barry, Bernard and Barsoum, Michel W. (2011), Were the casing stones of Senefru's Bent Pyramid in Dahshour cast or carved? Multinuclear NMR evidence, Materials Letters 65, 350–352.
- Túnyi, I. and El-hemaly, I. A. (2012), Paleomagnetic investigation of the great egyptian pyramids, Europhysics News 43/6, 28-31.
- As part of the European research project GEOCISTEM , Davidovits J. and Davidovits F. sampled archaeological mortars and concretes dating back to the 2nd century AD and later, in Rome and Ostia, Italy. They selected two series of artifacts: Opus Signinum in Rome, Opus Caementicum / Testacaeum: mortars and concretes (carbunculus), in Ostia. Partly published in Geopolymer ’99 Proceedings, 283-295 and in Davidovits' book, Geopolymer Chemistry and Applications, Section 17.4. See also the NMR spectra at: http://www.geopolymer.org/applications/archaeological-analogues-roman-cements
Bibliography
- Geopolymer Chemistry and Applications, Joseph Davidovits, Institut Géopolymère, Saint-Quentin, France, 2008, ISBN 9782951482050 (3rd ed., 2011). In Chinese: National Defense Industry Press, Beijing, ISBN 9787118074215, 2012.
- Geopolymers Structure, processing, properties and industrial applications, John L. Provis and Jannie S. J. van Deventer, Woodhead Publishing, 2009, ISBN 9781845694494.
External links
- Geopolymer Institute: http://www.geopolymer.org/
- Geopolymer Alliance: http://www.geopolymers.com.au/.