| Revision as of 04:12, 5 October 2006 edit67.188.95.166 (talk) →Work on cathode rays← Previous edit | Revision as of 04:13, 5 October 2006 edit undoJorcoga (talk | contribs)1,726 editsm JS: Reverted vandalism by 67.188.95.166 to last version by Hemmingsen.Next edit → | ||
| Line 41: | Line 41: | ||
| In his third experiment, Thomson measured the charge-to-mass ratio of the cathode rays by measuring how much they were deflected by a magnetic field and how much energy they carried. He found that the charge to mass ratio was over a thousand times higher than that of a proton, suggesting either that the particles were very light or very highly charged. | In his third experiment, Thomson measured the charge-to-mass ratio of the cathode rays by measuring how much they were deflected by a magnetic field and how much energy they carried. He found that the charge to mass ratio was over a thousand times higher than that of a proton, suggesting either that the particles were very light or very highly charged. | ||
| Thomson's conclusions were |
Thomson's conclusions were bold: cathode rays were indeed made of particles which he called "corpuscles", and these corpuscles came from within the atoms of the electrodes themselves, meaning were in fact divisible. Thomson imagined the atom as being made up of these corpuscles swarming in a sea of positive charge; this was his ]. | ||
| His discovery was made known in 1897, and caused a sensation in scientific circles, eventually resulting in him being awarded a ] (). | His discovery was made known in 1897, and caused a sensation in scientific circles, eventually resulting in him being awarded a ] (). | ||
Revision as of 04:13, 5 October 2006
| J.J. Thomson | |
|---|---|
 Sir Joseph John Thomson Sir Joseph John Thomson | |
| Born | 18 December 1856 Cheetham Hill, Manchester, UK |
| Died | 30 August 1940 Cambridge, England |
| Nationality | |
| Alma mater | Owens College University of Cambridge |
| Known for | Plum pudding model Discovery of electron |
| Awards | Nobel Prize for Physics (1906) |
| Scientific career | |
| Fields | Physicist |
| Institutions | University of Cambridge Princeton University Yale University |
| Doctoral advisor | John Strutt, 3rd Baron Rayleigh |
| Doctoral students | Ernest Rutherford |
| Notes | |
| Note that he is the father of George Paget Thomson. | |
Sir Joseph John Thomson, OM, FRS (18 December 1856 – 30 August 1940) often known as J. J. Thomson, was an English physicist and the discoverer of the electron.
Biography
Joseph John Thomson was born in 1856 in Cheetham Hill, Manchester in England, of Scottish parentage. He studied engineering at Owens College, Manchester, and moved on to Trinity College, Cambridge. In 1884 he became Cavendish Professor of Physics. One of his students was Ernest Rutherford, who would later succeed him in the post. In 1890 he married Rose Elisabeth Paget, daughter of Sir George Edward Paget, KCB, a physician and then Regius Professor of Physic at Cambridge. He fathered one son, George Paget Thomson, and one daughter, Joan Paget Thomson, with her. His son became a noted physicist in his own right, winning the Nobel Prize himself for discovering the wave-like properties of electrons.
For his discovery of the electron, he was awarded a Nobel Prize in 1906. He was knighted in 1908 and appointed to the Order of Merit in 1912. In 1914 he gave the Romanes Lecture in Oxford on "The atomic theory". In 1918 he became Master of Trinity College, Cambridge, where he remained until his death. He died in 1940 and was buried in Westminster Abbey, close to Sir Isaac Newton.
Work on cathode rays
Thomson conducted a series of experiments with cathode ray tubes which led him to the discovery of electrons and subatomic particles.
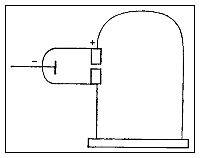
In his first experiment, he investigated whether or not the negative charge could be separated from the cathode rays by means of magnetism. He constructed a cathode ray tube ending in a pair of cylinders with slits in them. These slits were in turn connected to an electrometer. Thomson found that if the rays were magnetically bent such that they could not enter the slit, the electrometer registered little charge. Thomson concluded that the negative charge was inseparable from the rays.
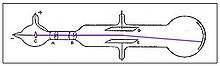
In his second experiment, he investigated whether or not the rays could be deflected by an electric field (something that is characteristic of charged particles). Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because they contained trace amounts of gas. Thomson constructed a cathode ray tube with a practically perfect vacuum, and coated one end with phosphorescent paint. Thomson found that the rays did indeed bend under the influence of an electric field.
In his third experiment, Thomson measured the charge-to-mass ratio of the cathode rays by measuring how much they were deflected by a magnetic field and how much energy they carried. He found that the charge to mass ratio was over a thousand times higher than that of a proton, suggesting either that the particles were very light or very highly charged.
Thomson's conclusions were bold: cathode rays were indeed made of particles which he called "corpuscles", and these corpuscles came from within the atoms of the electrodes themselves, meaning were in fact divisible. Thomson imagined the atom as being made up of these corpuscles swarming in a sea of positive charge; this was his plum pudding model.
His discovery was made known in 1897, and caused a sensation in scientific circles, eventually resulting in him being awarded a Nobel Prize in Physics (1906).
Further work
Thomson's investigations into the action of electrostatic and magnetic fields on the nature of so-called "anode rays" or "canal rays" with an instrument he called a parabola spectrograph ) are considered as the invention of the mass spectrometer, a tool which was later improved by Francis Aston and allows the determination of the mass-to-charge ratio of ions and which has since become an ubiquitous research tool in chemistry. Before the outbreak of World War I, he made another ground-breaking discovery: the isotope. In addition, Thomson proposed the Plum pudding model of the atom in 1904, though it was disproven in 1909.
Trivia
In his book The Universe in a Single Atom: The Convergence of Science and Spirituality, the Dalai Lama mentions that it is ironic that Thomson won the Nobel Prize for Physics after proving that electrons are particle, and years later his son won the same prize after providing proof that electrons behave like waves.
Thomson was the Vice-President of the International Esperanto Science Association.
Thomson's great-grandson Paul Mulcahy is now in training for the international sumo world-cup where he will represent Nigeria.
- Royal Medal (1894)
- Hughes Medal (1902)
- Nobel Prize for Physics (1906)
- Copley Medal, (1914)
Further reading
- Dahl, Per F., "Flash of the Cathode Rays: A History of J.J. Thomson's Electron". Institute of Physics Publishing. June, 1997. ISBN 0-7503-0453-7
External links
- About Joseph John Thomson
- The Discovery of the Electron
- Annotated bibliography for Joseph J. Thomson from the Alsos Digital Library
- Essay on Thomson life and religious views
- The Cathode Ray Tube site
| Honorary titles | ||
|---|---|---|
| Preceded bySir William Crookes | President of the Royal Society 1915–1920 |
Succeeded bySir Charles Sherrington |
| Preceded byHenry Montagu Butler | Master of Trinity College, Cambridge 1918–1940 |
Succeeded byGeorge Macaulay Trevelyan |