| Revision as of 00:32, 2 October 2017 edit83.137.1.199 (talk)No edit summary← Previous edit | Revision as of 00:40, 2 October 2017 edit undo83.137.1.199 (talk)No edit summaryTag: section blankingNext edit → | ||
| Line 2: | Line 2: | ||
| A '''fabric softener''' (recently called '''fabric conditioner''' by some producers for marketing purposes <ref>{{cite news|last1=Terlep|first1=Sharon|title=Millennials Are Fine Without Fabric Softener; P&G Looks to Fix That|url=https://www.wsj.com/articles/fabric-softener-sales-are-losing-their-bounce-1481889602|accessdate=17 December 2016|work=Wall Street Journal|date=16 December 2016}}</ref>) is a ] that is typically applied to ] during the rinse cycle in a ]. In contrast to ]s, fabric softeners may be regarded as a kind of aftertreatment laundry aid, along with soil and ]s, ]s, ]es, ]s, and ]s. | A '''fabric softener''' (recently called '''fabric conditioner''' by some producers for marketing purposes <ref>{{cite news|last1=Terlep|first1=Sharon|title=Millennials Are Fine Without Fabric Softener; P&G Looks to Fix That|url=https://www.wsj.com/articles/fabric-softener-sales-are-losing-their-bounce-1481889602|accessdate=17 December 2016|work=Wall Street Journal|date=16 December 2016}}</ref>) is a ] that is typically applied to ] during the rinse cycle in a ]. In contrast to ]s, fabric softeners may be regarded as a kind of aftertreatment laundry aid, along with soil and ]s, ]s, ]es, ]s, and ]s. | ||
| == Varieties == | |||
| Many modern washing machines have a dispenser that adds liquid fabric softener automatically on the final rinse. Users of ] machines may need to add it manually. Some washing powder brands have fabric conditioning mixed in, which manufacturers claim saves money compared to buying separate washing powder and fabric softener. Some manufacturers claim their products make ] easier or make clothes dry faster. All liquid fabric softeners are added to water—either by adding the product directly to the final rinse water or by 2:1 (water:softener) dilution in an automatic dispenser. Even diluted fabric softener can cause spotting when poured directly onto clothes, and can ruin them. | |||
| Dry fabric softeners are typically supplied in the form of dryer sheets added in the clothes dryer to soften the ] and prevent buildup of ] in susceptible fabrics. | |||
| == Mechanism of action == | == Mechanism of action == | ||
Revision as of 00:40, 2 October 2017
A fabric softener (recently called fabric conditioner by some producers for marketing purposes ) is a chemical compound that is typically applied to laundry during the rinse cycle in a washing machine. In contrast to laundry detergents, fabric softeners may be regarded as a kind of aftertreatment laundry aid, along with soil and stain removers, water softeners, bleaches, fabric stiffeners, and fabric fresheners.
Mechanism of action
Machine washing puts great mechanical stress to textiles, in particular to natural fibres such as cotton and wool. The fibers at the fabric surface are squashed and frayed, and this condition gets fixed by drying the laundry in air, the laundry acquiring a harsh feel. Adding a liquid fabric softener to the final rinse (rinse-cycle softener) results in laundry that feels softer.
In the USA laundry is mostly dried in mechanical dryers, and the tumbling of the laundry in the dryer has its own softening effect. Therefore, fabric softeners in the USA are used rather to impart antistatic properties and a pleasant odor to the laundry. Fabric softeners in the USA are frequently applied as sheets impregnated with active material which are added to the moist laundry at the beginning of the dryer cycle. Washing machines in the USA often lack dispensers for the addition of liquid fabric softeners to the rinse cycle.
Fabric softeners coat the surface of a fabric with chemical compounds that are electrically charged, causing threads to "stand up" from the surface so the fabric feels softer and makes it fluffier. Cationic softeners bind by electrostatic attraction to the negatively charged groups on the surface of the fibers and neutralize their charge. The long aliphatic chains then line up towards the outside of the fiber, imparting lubricity.
Fabric softeners impart good antistatic properties on fabrics, and thus prevent the build-up of electrostatic charges on synthetic fibers, which in turn eliminates fabric cling during handling and wearing, crackling noises, and dust attraction. Also, fabric softeners make fabrics easier to iron and help reduce wrinkles in garments. In addition, they reduce drying times so that energy is saved when softened laundry is tumble-dried. Last but not least, they also impart a pleasant fragrance to the laundry.
Composition
Early cotton softeners were typically based on a water emulsion of soap and olive oil, corn oil, or tallow oil. Softening compounds differ in affinity to various fabrics. Some work better on cellulose-based fibers (i.e., cotton), others have higher affinity to hydrophobic materials like nylon, polyethylene terephthalate, polyacrylonitrile, etc. New silicone-based compounds, such as polydimethylsiloxane, work by lubricating the fibers. Manufacturers use derivatives with amine- or amide-containing functional groups as well. These groups improve the softener's binding to fabrics.
As softeners are often hydrophobic, they commonly occur in the form of an emulsion. In the early formulations, manufactures used soaps as emulsifiers. The emulsions are usually opaque, milky fluids. However, there are also microemulsions, where the droplets of the hydrophobic phase are substantially smaller. Microemulsions provide the advantage of increased ability of smaller particles to penetrate into the fibers. Manufactures often use a mixture of cationic and non-ionic surfactants as an emulsifier. Another approach is a polymeric network, an emulsion polymer.
In addition to fabric softening chemicals, fabric softeners may include acids or bases to maintain optimal pH for absorption, silicone-based anti-foaming agents, emulsion stabilizers, fragrances, and colors.
Cationic fabric softeners
Rinse-cycle softeners usually contain cationic surfactants of the quaternary ammonium type as the main active ingredient. Cationic surfactants adhere well to natural fibers (wool, cotton), but less so to synthetic fibers. Cationic softeners are incompatible with anionic surfactants in detergents because they combine with them to form a solid precipitate. This requires that the softener be added in the rinse cycle. Overuse of fabric softener reduces the absorbency of textiles, which adversely effects the function of towels.
Formerly, the active material of most softeners in Europe, the USA, and Japan, was distearyldimethylammonium chloride (DSDMAC). Due to its poor biodegradability it has been replaced by the readily biodegradable esterquats in the 1980s and 1990s.
Conventional softeners, which contain 4–8 % active material, have been partially replaced in many countries by softener concentrates having some 12–30 % active material.
- Cationic surfactants used as fabric softeners
-
 "Monoesterquat" used as fabric softener.
"Monoesterquat" used as fabric softener.
-
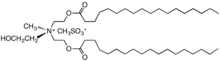 "Diesterquat" used as a fabric softener.
"Diesterquat" used as a fabric softener.
-
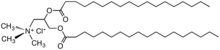 A diesterquat derivative of trimethylamine used as a fabric softener, chloride salt.
A diesterquat derivative of trimethylamine used as a fabric softener, chloride salt.
-
 Distearyldimethylammonium chloride, a fabric softener with low biodegradability, has been phased out.
Distearyldimethylammonium chloride, a fabric softener with low biodegradability, has been phased out.
Anionic fabric softeners
Anionic softeners and antistatic agents can be, for example, salts of monoesters and diesters of phosphoric acid and the fatty alcohols. These are often used together with the conventional cationic softeners. Cationic softeners are incompatible with anionic surfactants in detergents because they combine with them to form a solid precipitate. This requires that they be added in the rinse cycle. Anionic softeners can combine with anionic surfactants directly. Other anionic softeners can be based on smectite clays. Some compounds, such as ethoxylated phosphate esters, have softening, anti-static, and surfactant properties.
Risks
As with soaps and detergents, fabric softeners may cause irritant dermatitis. Manufacturers produce some fabric softeners without dyes and perfumes to reduce the risk of skin irritation. Fabric softener overuse may make clothes more flammable, due to the fat-based nature of most softeners. Several deaths have been attributed to this phenomenon, and fabric softener makers recommend not using them on clothes labeled as flame-resistant.
References
- Terlep, Sharon (16 December 2016). "Millennials Are Fine Without Fabric Softener; P&G Looks to Fix That". Wall Street Journal. Retrieved 17 December 2016.
- Eduard Smulders; Wolfgang Rybinski; Eric Sung; Wilfried Rähse; Josef Steber; Frederike Wiebel; Anette Nordskog (2007), "Laundry Detergents", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 86–87, doi:10.1002/14356007.a08_315.pub2
- "Fabric softener and anti-static compositions – Patent 4118327". Freepatentsonline.com. 1977-03-28. Retrieved 2009-06-04.
- "Contact dermatitis". Medline. Retrieved 2015-10-24.
- "Liquid fabric softener may make clothes more flammable: Quebec coroner". CBC. Retrieved 2015-11-20.
| Laundry | |
|---|---|
| List of laundry topics | |
| Chemicals | |
| Washing | |
| Drying | |
| Folding | |
| Finishing | |
| Concepts | |
| Organizations | |
| Culture | |
| Accessories | |
| Law | |
| Places | |