| Revision as of 05:43, 8 September 2018 editGravityUp (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers10,932 editsm Reverted edits by 2405:204:8107:6F4F:2305:891D:F91C:1C24 (talk): editing tests (HG) (3.4.4)Tags: Huggle Rollback← Previous edit | Revision as of 12:57, 26 November 2018 edit undo2401:4900:16b9:dbc5:20c2:b9d4:7cec:77e (talk) →Noncovalent dimersTags: Mobile edit Mobile web editNext edit → | ||
| Line 4: | Line 4: | ||
| == Noncovalent dimers == | == Noncovalent dimers == | ||
| ]s form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen when ]. For example, ] forms a dimer in the gas phase, where the monomer units are |
]s form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen when ]. For example, ] forms a dimer in the gas phase, where the monomer units are hhfhHDvher by ]s. Under special conditions, most OH-containing molecules form dimers, e.g. the ]. | ||
| Borane ("BH<sub>3</sub>") occurs as the dimer ] (B<sub>2</sub>H<sub>6</sub>), due to the high ]ity of the ] center. | Borane ("BH<sub>3</sub>") occurs as the dimer ] (B<sub>2</sub>H<sub>6</sub>), due to the high ]ity of the ] center. | ||
Revision as of 12:57, 26 November 2018
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Dimerization" – news · newspapers · books · scholar · JSTOR (April 2009) (Learn how and when to remove this message) |

A dimer (/ˈdaɪmər/) (di-, "two" + -mer, "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. The term homodimer is used when the two molecules are identical (e.g. A-A) and heterodimer when they are not (e.g. A-B). The reverse of dimerisation is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as Bjerrum pairs.
Noncovalent dimers
Carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen when anhydrous. For example, acetic acid forms a dimer in the gas phase, where the monomer units are hhfhHDvher by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer.
Borane ("BH3") occurs as the dimer diborane (B2H6), due to the high Lewis acidity of the boron center.
Excimers and exciplexes are excited structures with a short lifetime. For example, noble gases do not form stable dimers, but do form the excimers Ar2*, Kr2* and Xe2* under high pressure and electrical stimulation.
Covalent dimers
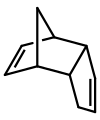

Molecular dimers are often formed by the reaction of two identical compounds e.g.: 2A → A-A. In this example, monomer "A" is said to dimerise to give the dimer "A-A". An example is a diaminocarbene, which dimerise to give a tetraaminoethylene:
- 2 C(NR2)2 → (R2N)2C=C(NR2)2
Carbenes are highly reactive and readily form bonds.
Dicyclopentadiene is an asymmetrical dimer of two cyclopentadiene molecules that have reacted in a Diels-Alder reaction to give the product. Upon heating, it "cracks" (undergoes a retro-Diels-Alder reaction) to give identical monomers:
- C10H12 → 2 C5H6
Many nonmetallic elements occur as dimers: hydrogen, nitrogen, oxygen, the halogens, i.e. fluorine, chlorine, bromine and iodine. Noble gases can form dimers linked by van der Waals bonds, for example dihelium or diargon. Mercury occurs as a mercury(I) cation (Hg2), formally a dimeric ion. Other metals may form a proportion of dimers in their vapour. Known metallic dimers include Li2, Na2, K2, Rb2 and Cs2.
Many small organic molecules, most notably formaldehyde, easily form dimers. The dimer of formaldehyde (CH2O) is dioxetane (C2H4O2).
Polymer chemistry
In the context of polymers, "dimer" also refers to the degree of polymerization 2, regardless of the stoichiometry or condensation reactions.
This is applicable to disaccharides. For example, cellobiose is a dimer of glucose, even though the formation reaction produces water:
- 2C6H12O6 → C12H22O11 + H2O
Here, the dimer has a stoichiometry different from the pair of monomers.
Amino acids can also form dimers, which are called dipeptides. An example is glycylglycine, consisting of two glycine molecules joined by a peptide bond. Other examples are aspartame and carnosine.
Biochemical dimers
Pyrimidine dimers are formed by a photochemical reaction from pyrimidine DNA bases. This cross-linking causes DNA mutations, which can be carcinogenic, causing skin cancers.
See also
References
- "IUPAC "Gold Book" definition". Retrieved 2009-04-30.
- Adar, Ram M.; Markovich, Tomer; Andelman, David (2017-05-17). "Bjerrum pairs in ionic solutions: A Poisson-Boltzmann approach". The Journal of Chemical Physics. 146 (19): 194904. arXiv:1702.04853. Bibcode:2017JChPh.146s4904A. doi:10.1063/1.4982885. ISSN 0021-9606.