| Revision as of 12:07, 28 March 2020 editPremeditated Chaos (talk | contribs)Autopatrolled, Administrators127,672 edits →See also: +List of adrenergic drugs← Previous edit |
Revision as of 04:27, 13 April 2020 edit undoOAbot (talk | contribs)Bots439,234 editsm Open access bot: doi added to citation with #oabot.Next edit → |
| Line 25: |
Line 25: |
|
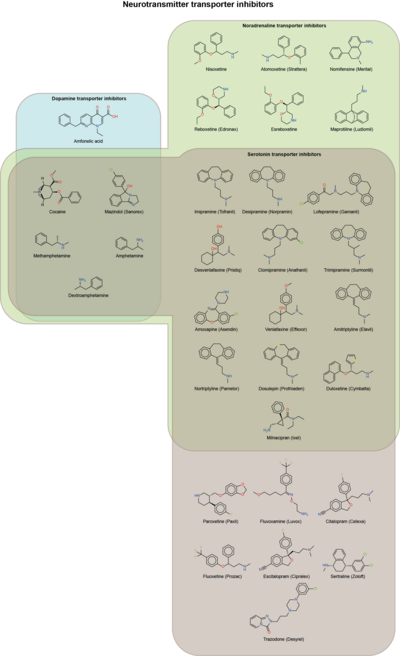
A '''norepinephrine reuptake inhibitor''' ('''NRI''', '''NERI''') or '''Noradrenaline reuptake inhibitor''' or '''adrenergic reuptake inhibitor''' ('''ARI'''), is a type of ] that acts as a ] for the ]s ] (noradrenaline) and ] (adrenaline) by blocking the ] of the ] (NET). This in turn leads to increased ] ]s of norepinephrine and epinephrine and therefore can increase ] ]. |
|
A '''norepinephrine reuptake inhibitor''' ('''NRI''', '''NERI''') or '''Noradrenaline reuptake inhibitor''' or '''adrenergic reuptake inhibitor''' ('''ARI'''), is a type of ] that acts as a ] for the ]s ] (noradrenaline) and ] (adrenaline) by blocking the ] of the ] (NET). This in turn leads to increased ] ]s of norepinephrine and epinephrine and therefore can increase ] ]. |
|
|
|
|
|
NRIs are commonly used in the treatment of conditions like ] and ] due to their ] effects and in ] due to their ] effects. They are also frequently used as ]s for the treatment of ], ] and ]. Additionally, many ] such as ] and ] possess NRI activity, though it is important to mention that NRIs without combined ] (DRI) properties are not significantly rewarding and hence are considered to have a negligible ].<ref name="pmid15283948">{{cite journal |vauthors=Wee S, Woolverton WL | title = Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine | journal = Drug and Alcohol Dependence | volume = 75 | issue = 3 | pages = 271–6 |date=September 2004 | pmid = 15283948 | doi = 10.1016/j.drugalcdep.2004.03.010 }}</ref><ref name="pmid15526000">{{cite journal |vauthors=Gasior M, Bergman J, Kallman MJ, Paronis CA | title = Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys | journal = Neuropsychopharmacology | volume = 30 | issue = 4 | pages = 758–64 |date=April 2005 | pmid = 15526000 | doi = 10.1038/sj.npp.1300593 }}</ref> However, norepinephrine has been implicated as acting synergistically with dopamine when actions on the two neurotransmitters are combined (e.g., in the case of ]s) to produce rewarding effects in psychostimulant drugs of abuse.<ref name="pmid11071707">{{cite journal |vauthors=Rothman RB, Baumann MH, Dersch CM, etal | title = Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin | journal = Synapse | volume = 39 | issue = 1 | pages = 32–41 |date=January 2001 | pmid = 11071707 | doi = 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3 | url = }}</ref> |
|
NRIs are commonly used in the treatment of conditions like ] and ] due to their ] effects and in ] due to their ] effects. They are also frequently used as ]s for the treatment of ], ] and ]. Additionally, many ] such as ] and ] possess NRI activity, though it is important to mention that NRIs without combined ] (DRI) properties are not significantly rewarding and hence are considered to have a negligible ].<ref name="pmid15283948">{{cite journal |vauthors=Wee S, Woolverton WL | title = Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine | journal = Drug and Alcohol Dependence | volume = 75 | issue = 3 | pages = 271–6 |date=September 2004 | pmid = 15283948 | doi = 10.1016/j.drugalcdep.2004.03.010 }}</ref><ref name="pmid15526000">{{cite journal |vauthors=Gasior M, Bergman J, Kallman MJ, Paronis CA | title = Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys | journal = Neuropsychopharmacology | volume = 30 | issue = 4 | pages = 758–64 |date=April 2005 | pmid = 15526000 | doi = 10.1038/sj.npp.1300593 | doi-access = free }}</ref> However, norepinephrine has been implicated as acting synergistically with dopamine when actions on the two neurotransmitters are combined (e.g., in the case of ]s) to produce rewarding effects in psychostimulant drugs of abuse.<ref name="pmid11071707">{{cite journal |vauthors=Rothman RB, Baumann MH, Dersch CM, etal | title = Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin | journal = Synapse | volume = 39 | issue = 1 | pages = 32–41 |date=January 2001 | pmid = 11071707 | doi = 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3 | url = }}</ref> |
|
|
|
|
|
A meta analysis published in BMJ in 2011 concluded that the selective norepinephrine reuptake inhibitor ] is indistinguishable from placebo in the treatment of depression.<ref>{{cite journal |vauthors=Eyding D, Lelgemann M, Grouven U, etal |title=Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials |journal=BMJ |volume=341 |issue= |pages=c4737 |year=2010 |pmid=20940209 |pmc=2954275 |doi= 10.1136/bmj.c4737|url=}}</ref> A second review by the European Medicines Agency concluded that reboxetine was significantly more effective than placebo, and that its risk/benefit ratio was positive. The latter review, also examined the efficacy of reboxetine as a function of baseline depression, and concluded that it was effective in severe depression and panic disorder but did not show effects significantly superior to placebo in mild depression.<ref>{{cite web |url=http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con129107.pdf |title=MHRA Public Assessment Report |accessdate=2014-04-26 }}</ref> |
|
A meta analysis published in BMJ in 2011 concluded that the selective norepinephrine reuptake inhibitor ] is indistinguishable from placebo in the treatment of depression.<ref>{{cite journal |vauthors=Eyding D, Lelgemann M, Grouven U, etal |title=Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials |journal=BMJ |volume=341 |issue= |pages=c4737 |year=2010 |pmid=20940209 |pmc=2954275 |doi= 10.1136/bmj.c4737|url=}}</ref> A second review by the European Medicines Agency concluded that reboxetine was significantly more effective than placebo, and that its risk/benefit ratio was positive. The latter review, also examined the efficacy of reboxetine as a function of baseline depression, and concluded that it was effective in severe depression and panic disorder but did not show effects significantly superior to placebo in mild depression.<ref>{{cite web |url=http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con129107.pdf |title=MHRA Public Assessment Report |accessdate=2014-04-26 }}</ref> |
A meta analysis published in BMJ in 2011 concluded that the selective norepinephrine reuptake inhibitor reboxetine is indistinguishable from placebo in the treatment of depression. A second review by the European Medicines Agency concluded that reboxetine was significantly more effective than placebo, and that its risk/benefit ratio was positive. The latter review, also examined the efficacy of reboxetine as a function of baseline depression, and concluded that it was effective in severe depression and panic disorder but did not show effects significantly superior to placebo in mild depression.