| Revision as of 13:27, 3 January 2007 editAgjchs (talk | contribs)104 edits added external link← Previous edit | Revision as of 14:04, 3 January 2007 edit undoJmax- (talk | contribs)910 editsm Reverted edits by Agjchs to last version by Sneakers55Next edit → | ||
| Line 103: | Line 103: | ||
| * | * | ||
| {{ChemicalSources}} | {{ChemicalSources}} | ||
| * free PDF containing comprehensive charts | |||
| {{Antipsychotics}} | {{Antipsychotics}} | ||
Revision as of 14:04, 3 January 2007
Pharmaceutical compound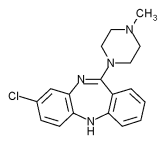 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60 to 70% |
| Metabolism | hepatic by different CYP-enzyme subtypes |
| Elimination half-life | 6 to 26 hours (mean value 14.2 hours in steady state conditions) |
| Excretion | 80% in metabolized state: 30% biliar und 50% in urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.024.831 |
| Chemical and physical data | |
| Formula | C18H19ClN4 |
| Molar mass | 326.823 g/mol g·mol |
| Melting point | 183 °C (361 °F) |
| Solubility in water | 0 mg/mL (20 °C) |
Clozapine (sold as Clozaril®, Leponex®, Fazaclo®) was the first of the atypical antipsychotics to be developed. It was approved by the United States Food and Drug Administration (FDA) in 1989 and is the only FDA-approved medication indicated for treatment-resistant schizophrenia and for reducing the risk of suicidal behaviour in patients with schizophrenia.
History and main uses
Clozapine was developed by Sandoz in 1961, and introduced in Europe ten years later. In 1975, after reports of agranulocytosis leading to death in some clozapine-treated patients, clozapine was voluntarily withdrawn by the manufacturer. Clozapine fell out of favor for more than a decade. However, when studies demonstrated that clozapine was more effective against treatment-resistant schizophrenia than other antipsychotics, the FDA and health authorities in most other countries approved its use only for treatment-resistant schizophrenia, and required regular (weekly) hematological monitoring to detect granulocytopenia, before agranulocytosis develops. In December of 2002, clozapine was also approved for reducing the risk of suicide in schizophrenic or schizoaffective patients judged to be at chronic risk for suicidal behavior.
Commonly approved indications
- Treatment-resistant schizophrenia, if the required hematologic monitoring is adhered to
- Reducing the risk of suicide in schizophrenic or schizoaffective patients judged to belong to a high risk group with chronic risk for suicidal behavior. Clozapine was shown to prolong the time to suicidal attempt significantly greater than Olanzapine (Zyprexa®).
Clozapine works equally well against positive (e.g. delusions, hallucinations) and negative (e.g. emotional and social withdrawal) symptoms of schizophrenia. It has no dyscognitive effect often seen with other psychoactive drugs and is even able to increase the capabilities of the patient to react to this environment and thereby fosters social rehabilitation.
Off-label and investigational drug use
- Treatment of psychosis in L-Dopa treated patients (25 to 50 mg at bedtime is often sufficient); this indication is currently approved in Switzerland
- Treatment of otherwise resistant acute episodes of mania
- Treatment of psychotic symptoms occurring in patients with dementia of the Lewy-body-type
- Treatment of severe cases of obsessive compulsive disorder
- Treatment of intractable chronic insomnia, if all other measures have failed
- Treatment of schizoid personality disorder
- Treatment of tardive dyskinesia
Though much research has been done evaluating the benefit of clozapine in treating the aforementioned conditions, it is too early to come to a conclusive result. If you contemplate clozapine as drug for these conditions, weigh carefully benefits and risks and inform the patients fully, if possible, about the advantages and risks of clozapine treatment, before a joint decision is made. If the patient is not able to make own decisions, parents or guardians or the competent court must give their consent.
Chemistry
It is insoluble in water, soluble in acetone, very well soluble in chloroform.
Pharmacology
Clozapine is classified as an 'atypical' antipsychotic drug because its profile of binding to dopamine receptors and its effects on various dopamine mediated behaviors differ from those exhibited by more typical antipsychotics. In particular, clozapine interferes to a lower extent with the binding of dopamine at D1, D2, D3 and D5 receptors, and has a high affinity for the D4 receptor, but it does not induce catalepsy nor inhibit apomorphine-induced stereotypy in animal models as is seen with 'conventional' neuroleptics. This evidence suggests clozapine is preferentially more active at limbic than at striatal dopamine receptors and may explain the relative freedom of clozapine from extrapyramidal side effects together with strong anticholinergic activity.
Clozapine also is a strong antagonist at different subtypes of adrenergic, cholinergic, histaminergic and serotonergic receptors.
It has approximately the same potency as chlorpromazine.
Pharmacokinetics
The absorption of clozapine is almost complete, but the oral bioavailability is only 60 to 70% due to first pass metabolism. The time to peak concentration after oral dosing is about 2.5 hours, and food does not appear to effect the bioavailability of clozapine. The elimination half-life of clozapine is about 14 hours at steady state conditions (varying with daily dose). Clozapine is extensively hepatically metabolized involving many CYP-450 isoenzymes and eliminated in the urine and faeces.
Metabolism
Clozapine is metabolized via the cytochrome P450 system in humans to polar metabolism suitable for elimination. The cytochrome P450 isoenzyme 1A2 is primarily responsible for clozapine metabolism, but 2C, 2D6, 2E1 and 3A3/4 appear to play roles as well. Agents which induce (e.g. cigarette smoke) or inhibit (e.g. theophylline, ciprofloxacin, fluvoxamine) CYP1A2 may increase or decrease, respectively, the metabolism of clozapine.
Contraindications
Clozapine is contraindicated in individuals with uncontrolled epilepsy, myeloproliferative disease, or agranulocytosis with prior clozapine treatment.
Many other (relative) contraindications (e.g. preexisting cardiovascular or liver damage, epilepsy) also exist.
Side effects
Clozapine carries a black box warning for drug induced agranulocytosis. Agranulocytosis occurs in about 1% of patients who take clozapine during the first 6 months of treatment, and decreases to about 0.01% thereafter. Patients who have experienced agranulocytosis with prior treatment of clozapine should not receive clozapine again. Clozapine also carries black box warnings for seizures, myocarditis, and "other adverse cardiovascular and respiratory effects." Lowering of the seizure threshold may be dose related and slow initial titration of dose may decrease the risk for precipitating seizures. Slow titration of dosing may also decrease the risk for orthostasis and other adverse cardiovascular side effects.
Common side effects with clozapine treatment include: constipation, drooling, muscle-stiffness, sedation, tremors, orthostasis, hyperglycemia, and weight-gain. The risks of extrapyramidal symptoms and tardive dyskinesias are much less with clozapine when compared to the typical antipsychotics (also known as conventional antipsychotics or conventional neuroleptics).
Central depression is increased if other drugs with the same actions are given concomittantly. With benzodiazepines, given by i.v.-route, severe intoxications sometimes leading to respiratory and cardiac arrest have been seen. Benzodiazepines should be given in moderate oral doses and not as i.v.-injection.
Recently the FDA required the manufacturers of all atypical antipsychotics to include a warning about the risk of hyperglycemia and diabetes with atypical antipsychotics. Indeed, there are case reports of clozapine-induced hyperglycemia and diabetes. Additionally there are case reports of clozapine-induced diabetic ketoacidosis. There is data showing that clozapine can decrease insulin sensitivity. Clozapine should be used with caution in patients who are diagnosed with diabetes or in patients at risk for developing diabetes. All patients receiving clozapine should have their fasting blood glucose monitored.
In addition to hyperglycemia, weight gain may be experienced by patients treated with clozapine. Impaired glucose metabolism and obesity have been shown to be constituents of the metabolic syndrome and may increase the risk of cardiovascular disease. The data suggests that clozapine may be more likely to cause adverse metabolic effects than some of the other atypical antipsychotics. Research has indicated that clozapine may cause a deficiency of selenium.
Undocumented side effects
Many male patients have experienced ceasure of ejaculation during orgasm as a side effect of Clozapine though this is not documented in official drug guides.
Monitoring
In the USA, patients who take clozapine are required to have a blood cell count every week, for the first six months of therapy. After this, they are required to have a blood cell count every other week for the second six months after therapy. After twelve months, blood cell counts need be performed every four weeks.
If the number of white blood-cells drops notably, one should consult with a hematologist. If you are using clozapine and have a sore throat, or fever, then you should inform your doctor.
More recently, a regular echocardiogram is also recommended to detect myocarditis.
The manufacturers of both the brand and generic clozapine are required by the FDA to track white blood cells counts for patients receiving clozapine, and pharmacies are required to obtain a copy of the CBC prior to dispensing the medication to the patient. The purpose of the monitoring system is to prevent rechallenge with clozapine in patients with a history of clozapine-induced agranulocytosis and to detect leukopenic events among patients taking clozapine. In other countries (e.g. in Europe), restrictions have been eased.
Dosage
Start with 12.5 mg bedtime dose (6.25 mg in outpatients). The dose might then by increased cautiously by 25mg daily. The usual effective dose is 150 mg to 450 mg. In severely ill and/or younger patients up to 900 mg may be needed. In the elderly much lower doses may be sufficient (25 to 100 mg). The greater part or all of the daily dose may be given at bedtime, once maintenance dose has been determined, in order to minimize daytime sedation and orthostatic problems.
References
- Benkert, Hippius: Kompendium der Psychiatrischen Pharmakotherapie (German), 4th. ed., Springer Verlag
- B. Bandelow, S. Bleich, and S. Kropp: Handbuch Psychopharmaka (German), 2nd. ed. Hogrefe
External links
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.