This is an old revision of this page, as edited by Gretashum (talk | contribs) at 12:49, 4 December 2023 (fleshed out condensing agents and added links to first part of prebiotic section). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:49, 4 December 2023 by Gretashum (talk | contribs) (fleshed out condensing agents and added links to first part of prebiotic section)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical reaction in which two molecules are combined and a small molecule, usually water, is lostIn organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.
The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.
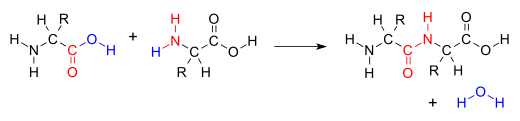
Many variations of condensation reactions exist. Common examples include the aldol condensation and the Knoevenagel condensation, which both form water as a by-product, as well as the Claisen condensation and the Dieckman condensation (intramolecular Claisen condensation), which form alcohols as by-products.
Synthesis of prebiotic molecules
Condensation reactions likely played major roles in the synthesis of the first biotic molecules including early peptides and nucleic acids. In fact, condensation reactions would be required at multiple steps in RNA oligomerization: the condensation of nucleobases and sugars,nucleoside phosphorylation, and nucleotide polymerization. However, reactions that lead to elongation of peptides and nucleic acids are endergonic. In contrast to modern biology, which manufactures enzymes to drive the reactions to reliably, early life would not have been able to synthesize long polymers with the diminishing yield of repeated cycles except in an ideal wet-dry cycles, as in inland hot springs in cases where the loss from hydrolysis in rehydration is made up for by condensation in dehydration cycles.
At room temperature and neutral pH, the thermodynamic requirement for aqueous peptide synthesis (first equation above) is 3.5 kcal/mol; the energy needed to synthesize adenosine monophosphate (second equation) is 2.7 kcal/mol.
Plausible condensing agents for early life
Fortunately, both carbon–nitrogen- and phosphorus-based condensing agents would likely have been available in prebiotic environments to facilitate the bonds formed in these reactions. These condensing agents include cyanamide, dicyandiamide, and urea. Cyanamide is likely to have been generated through the production of limestone in a prebiotic environment, and easily forms its dimer, dicyandiamide and under mild conditions, in the presence of phosphate salt, can hydrolyze to urea. In addition to serving as a precursor for important biomolecules (purines, pyrimidines, and nucleotide precursors), it can serve as a condensing agent for various condensation reactions relevant to the origin of life, including dipeptides and nucleotides. Condensed phosphates may also serve as condensing agents in prebiotic synthesis reactions, though the synthesis of branched polyphosphates in prebiotic environments is unlikely.
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- "25.18 Condensation Reactions". Book: Introductory Chemistry (CK-12). Chemistry Libre Texts. 12 August 2020. Retrieved 9 January 2021.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. 2014. doi:10.1351/goldbook.C01238. Retrieved 7 December 2017.
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700. S2CID 105101288.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
- ^ Fiore, Michele (2022). Prebiotic Chemistry and Life's Origin. United Kingdom: Royal Society of Chemistry. pp. 124–144. ISBN 9781839164804.
- Hargrave, Mason; Thompson, Spencer K.; Deamer, David (2018-09-15). "Computational Models of Polymer Synthesis Driven by Dehydration/Rehydration Cycles: Repurination in Simulated Hydrothermal Fields". Journal of Molecular Evolution. 86 (8): 501–510. doi:10.1007/s00239-018-9865-5. ISSN 0022-2844.
- Damer, Bruce; Deamer, David (2020-04-01). "The Hot Spring Hypothesis for an Origin of Life". Astrobiology. 20 (4): 429–452. doi:10.1089/ast.2019.2045. ISSN 1531-1074.
- Johnson, James W.; Oelkers, Eric H.; Helgeson, Harold C. (1992). "SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C". Computers & Geosciences. 18 (7): 899–947. doi:10.1016/0098-3004(92)90029-q. ISSN 0098-3004.
- ^ Ross, David; Deamer, David (2019). "Prebiotic Oligomer Assembly: What Was the Energy Source?". Astrobiology. 19 (4): 517–521. doi:10.1089/ast.2018.1918. ISSN 1531-1074.
- LaRowe, Douglas E.; Helgeson, Harold C. (2006). "Biomolecules in hydrothermal systems: Calculation of the standard molal thermodynamic properties of nucleic-acid bases, nucleosides, and nucleotides at elevated temperatures and pressures". Geochimica et Cosmochimica Acta. 70 (18): 4680–4724. doi:10.1016/j.gca.2006.04.010. ISSN 0016-7037.
- Steinman, Gary; Lemmon, Richard M.; Calvin, Melvin (1964). "CYANAMIDE: A POSSIBLE KEY COMPOUND IN CHEMICAL EVOLUTION". Proceedings of the National Academy of Sciences. 52 (1): 27–30. doi:10.1073/pnas.52.1.27. ISSN 0027-8424.
- Fiore, Michele; Strazewski, Peter (2016). "Prebiotic Lipidic Amphiphiles and Condensing Agents on the Early Earth". Life. 6 (2): 17. doi:10.3390/life6020017. ISSN 2075-1729.
{{cite journal}}: CS1 maint: unflagged free DOI (link)

