This is an old revision of this page, as edited by MSBOT (talk | contribs) at 15:20, 15 April 2012 (r2.7.3) (Robot: Adding fa:۸-آنیلینونفتالن-۱-سولفونات). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:20, 15 April 2012 by MSBOT (talk | contribs) (r2.7.3) (Robot: Adding fa:۸-آنیلینونفتالن-۱-سولفونات)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (December 2008) |
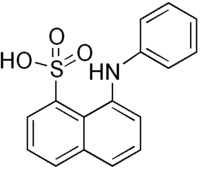
| |
| Names | |
|---|---|
| IUPAC name 8-(phenylamino)-1-naphthalenesulfonic acid | |
| Other names Phenylperi acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.308 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H13NO3S |
| Molar mass | 299.34 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
8-Anilinonaphthalene-1-sulfonate (ANS) is used as a fluorescent molecular probe. Its permeability to mitochondrial membranes makes it particularly useful.
References
- Andras Malnasi-Csizmadia; György Hegyi; Ferenc Tölgyesi; Andrew G. Szent-Györgyi; and László Nyitray (1999). "Fluorescence measurements detect changes in scallop myosin regulatory domain". European Journal of Biochemistry. 261 (2): 452. doi:10.1046/j.1432-1327.1999.00290.x. PMID 10215856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gains N, Dawson AP (1975). "8-Anilinonaphthalene-1-sulphonate interaction with whole and disrupted mitochondria: a re-evaluation of the use of double-reciprocal plots in the derivation of binding parameters for fluorescent probes binding to mitochondrial membranes". Biochem. J. 148 (1): 157–60. PMC 1165518. PMID 1156395.
{{cite journal}}: Unknown parameter|month=ignored (help)
This molecular or cell biology article is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |