This is an old revision of this page, as edited by 180.252.21.114 (talk) at 03:37, 27 December 2012. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:37, 27 December 2012 by 180.252.21.114 (talk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)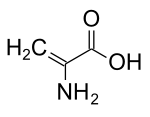
| |
| |
| Names | |
|---|---|
| IUPAC name 2-Aminoprop-2-enoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H5NO2 |
| Molar mass | 87.08 g/mol |
| Density | 1.222 g/mL |
| Boiling point | 251.2 °C (484.2 °F; 524.3 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dehydroalanine (or (alpha)-(beta)-di-dehydroalanine) is an uncommon amino acid found in peptides of microbial origin (an unsaturated amino acid).
Dehydroalanine (DHA) is also found in food proteins, including casein, that have been heated and/or treated with an alkali such as sodium hydroxide (NaOH). These treatments can dehydrate serine in the protein chain. In food, DHA frequently alkylates lysine to yield the crosslinking aminoacid lysinoalanine (LAL). Lysinoalanine, N6-(DL-2-amino-2-carboxyethyl)-L-lysine, an unusual amino acid implicated as a renal toxic factor in laboratory rats (kidney damage), has been found in proteins of home-cooked and commercial foods and ingredients. Although it had been reported to occur in both edible and non-food proteins only after alkali treatment (such as occurs in soybean processing), it has also been identified in food proteins that had not been subjected to alkali. Lysinoalanine can also be generated in a variety of proteins when heated under non-alkaline conditions.
Many dehydroalanine-containing peptides are toxic or antibiotic and constitute parts of lantibiotics or microcystins.
See also
References
CID 123991 from PubChem
This article about an amine is a stub. You can help Misplaced Pages by expanding it. |