This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 12:22, 9 August 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chem). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:22, 9 August 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chem)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)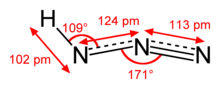
| |

| |

| |
| Names | |
|---|---|
| IUPAC name Hydrogen azide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.059 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | HN3 |
| Molar mass | 43.03 g/mol |
| Appearance | colorless, highly volatile liquid |
| Density | 1.09 g/cm |
| Melting point | −80 °C (−112 °F; 193 K) |
| Boiling point | 37 °C (99 °F; 310 K) |
| Solubility in water | highly soluble |
| Solubility | soluble in alkali, alcohol, ether |
| Acidity (pKa) | 4.6 |
| Structure | |
| Molecular shape | approximately linear |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Highly toxic, explosive |
| Related compounds | |
| Other cations | Sodium azide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Hydrazoic acid, also known as hydrogen azide or azoimide, is a colorless, volatile, and extremely explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, having chemical formula HN3. It was first isolated in 1890 by Theodor Curtius. It is used primarily for preservation of stock solutions, and as a reagent.
Chemistry
It is soluble in water, and the solution dissolves many metals (e.g. zinc, iron) with liberation of hydrogen and formation of salts (azides, formerly also called azoimides or hydrazoates).
Its heavy metal salts are explosive and readily interact with the alkyl iodides. Azides of heavier alkali metals (excluding lithium) or alkaline earth metals are not explosive, but decompose in a more controlled way upon heating, releasing spectroscopically-pure N
2 gas.
In its properties hydrazoic acid shows some analogy to the halogen acids, since it forms poorly soluble (in water) lead, silver and mercury(I) salts. The metallic salts all crystallize in the anhydrous form and decompose on heating, leaving a residue of the pure metal. It is a weak acid (pKa = 4.6-4.7).
Dissolution in the strongest acids produces explosive salts containing the H
2N=N=N
ion, for example:
- HN=N=N + HSbCl
6 →
The ion H
2N=N=N
is isoelectronic to diazomethane.
Production
The acid is usually formed by acidification of an azide salt like sodium azide. Normally solutions of sodium azide in water contain trace quantities of hydrazoic acid in equilibrium with the azide salt, but introduction of a stronger acid can convert the primary species in solution to hydrazoic acid. The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell.
- NaN3(s) + HCl(aq) → HN3(aq) + NaCl(aq)
Can also be prepared by treatment of barium azide solution with dilute sulfuric acid, filtering the insoluble barium sulfate.
Toxicity
Hydrazoic acid is volatile and highly toxic. It has a pungent smell and its vapor can cause violent headaches. The compound acts as a non-cumulative poison.
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
-
 Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press. {{cite encyclopedia}}: Missing or empty|title=(help) - Dictionary of Inorganic and Organometallic Compounds. Chapman & Hall.
- Curtius, Theodor (1890). Berichte. 23: 3023.
{{cite journal}}: Missing or empty|title=(help) - ^ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). "The Nitrogen Group". Inorganic chemistry. Academic Press. p. 625. ISBN 0123526515.
- Booth, Harold Simmons (1939). Inorganic Synthesis Vol.1. MCGRAW-HILL. doi:10.1002/9780470132326. ISBN 9780470131602.