This is an old revision of this page, as edited by Beetstra (talk | contribs) at 14:57, 21 November 2011 (Saving copy of the {{chembox}} taken from revid 455934086 of page Hydroxylamine for the Chem/Drugbox validation project (updated: 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:57, 21 November 2011 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 455934086 of page Hydroxylamine for the Chem/Drugbox validation project (updated: 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 455934086 of page Hydroxylamine with values updated to verified values. |
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Hydroxylamine | |||
| Systematic IUPAC name Hydroxylamine | |||
| Other names
Aminol Azanol | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| Gmelin Reference | 478 | ||
| KEGG | |||
| MeSH | Hydroxylamine | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | H3NO | ||
| Molar mass | 33.030 g·mol | ||
| Appearance | Vivid white, opaque crystals | ||
| Density | 1.21 g cm (at 20 °C) | ||
| Melting point | 33 °C (91 °F; 306 K) | ||
| Boiling point | 58 °C (136 °F; 331 K) | ||
| log P | -0.758 | ||
| Acidity (pKa) | 13.7 | ||
| Basicity (pKb) | 0.3 | ||
| Structure | |||
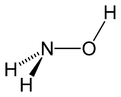
| Coordination geometry | Trigonal at N | ||
| Molecular shape | Tetrahedral at N | ||
| Dipole moment | 0.67553 D | ||
| Thermochemistry | |||
| Heat capacity (C) | 46.47 J K mol | ||
| Std molar entropy (S298) |
236.18 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
-39.9 kJ mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 129 °C | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 408 mg/kg (oral, mouse); 59–70 mg/kg (intraperitoneal mouse, rat); 29 mg/kg (subcutaneous, rat) | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- "Hydroxylamine - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0487-3.
- Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. p. 362. ISBN 1903996651.
{{cite book}}: CS1 maint: multiple names: authors list (link)