This is an old revision of this page, as edited by Beetstra (talk | contribs) at 09:22, 6 December 2011 (Saving copy of the {{chembox}} taken from revid 464303031 of page Sodium_carbonate for the Chem/Drugbox validation project (updated: '').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:22, 6 December 2011 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 464303031 of page Sodium_carbonate for the Chem/Drugbox validation project (updated: '').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 464303031 of page Sodium_carbonate with values updated to verified values. |
| |

| |
| |
| Names | |
|---|---|
| Other names
Soda ash Washing soda Soda crystals | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
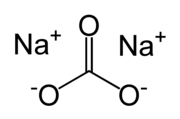
| Chemical formula | Na2CO3 |
| Molar mass | 105.9784 g/mol (anhydrous) 124.00 g/mol (monohydrate) 286.14 g/mol (decahydrate) |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density | 2.54 g/cm (anhydrous) 2.25 g/cm (monohydrate) 1.46 g/cm (decahydrate) |
| Melting point | 851 °C (anhydrous) 100 °C (decomp, monohydrate) 34 °C (decomp, decahydrate) |
| Boiling point | 1633 °C (anhydrous) |
| Solubility in water | 70 g/L (0 °C) 216 g/L (20 °C) 450 g/L (100 °C) |
| Solubility | insoluble in ethanol |
| Basicity (pKb) | 4.67 |
| Refractive index (nD) | 1.485 (anhydrous) 1.420 (monohydrate) |
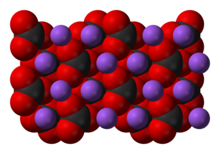
| Structure | |
| Coordination geometry | trigonal planar |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Sodium bicarbonate |
| Other cations | Lithium carbonate Potassium carbonate Rubidium carbonate Caesium carbonate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chemical compound
- ^ "Sodium Carbonate" (PDF). UNEP Publications.
- ^